Growing recognition of long-term COVID vaccination adverse reactions
Part III-ish: Consideration of SFN/POTS and treatment/management thoughts.
Apologies to readers. My previous post mentioned a look into the hypercatecholaminergic response in lieu of the information we have looked at so far when it comes to POTS and SFN. However this, along with a look into SFN/POTS and autoimmunity, will be saved for another post to limit the length of these articles.
Also, please note that the following information is intent on providing knowledge, and not to be used as medical information. Please always consult with your doctor for more information and treatment options.
Previous posts for those interested:
Small fiber neuropathy (SFN):
Postural orthostatic tachycardia syndrome (POTS):
In regards to POTS
It should be noted that POTS can fall under the umbrella of SFN if the symptoms are assumed to be neuropathic in nature. Although several of the cases presented yesterday noted confirmed cases of POTS, there were limited evidence of any form of neuropathy aside from the presentation of Raynaun’s Phenomenon in the Hermel, et al. case report.
However, this doesn’t discredit the possibility of an autoimmune-related pathology for these patients or even SFN, as damage to nerves responsible for autonomic function may contribute to the compensatory hyperactivation of the sympathetic system. Tachycardia is also considered to a be a possible sign of neuropathy.
Because no autoantibody testing appeared to have been conducted, and were vague in instances where testing did appear, we are left with gaps in knowledge. Note that, in a similar manner to that of SFN, several patients with POTS are noted to have autoantibodies that target adrenergic or RAS receptors. The targeting of adrenergic receptors would, again be suggestive of altered norepinephrine responses.
Ormiston, et al. elaborates on this point in their review:
There is also a growing body of evidence reporting the presence of antinuclear antibodies, antiphospholipid antibodies, α1, β1, and β2 adrenergic receptors antibodies, angiotensin 2 type 1 receptor antibodies, ganglionic N-type and P/Q-type acetylcholine receptor antibodies, opioid-like 1 receptor antibodies, and muscarinic M2 and M4 antibodies in patients with POTS.1 , 9 , 21, 22, 23, 24, 25 The presence of these antibodies, suggesting autoimmunity, may explain some dysautonomia symptoms (eg, tachycardia and elevated standing plasma norepinephrine levels) found in POTS.21
Note that several of the patients in the POTS case reports were provided beta-blockers, which prevent binding of catecholamines such as norepinephrine and epinephrine to β adrenergic receptors which reduced symptoms of tachycardia, and likely helps to attenuate some of the hyper sympathetic activation.
Overall, this raises the importance of conducting bloodwork and examining for autoantibodies which is likely not occurring. It may also be necessary to look for signs of SFN which patients may not recognize, such as loss of sensation or numbness in their extremities.
This too, raises a critical issue in the lack of antibody characterization. Consider that many studies just note the neutralization capacity of these antibodies, but rarely do they characterize the epitopes that these antibodies target, or whether they show cross-reactivity. This adds further issues to the antibody-only narrative that these vaccines have constructed.
What stands out in the case of POTS are the two adolescents in particular, who each experienced symptoms after their second or third dose. The prognosis here seems more concerning given the lack of evidence for full resolution in either case, especially in the case of the boy who had overlapping myocarditis, with both appearing to debilitate him for nearly a year.
Again, one of the questions that has yet to be elucidated is the strange presentation in adolescents when compared to adults. In several of these reports adults appear to experience symptoms after their first dose of a COVID vaccine (aside from the woman who had a prior COVID infection). We are dealing with limited case reports, and so other assessments may discredit this anomaly.
In the meantime, it again raises a point of whether this age-related discrepancy is due to difference between innate and adaptive immune responses in children compared to adults. If autoimmunity is argued, it raises a question of whether other prior priming scenarios may lead to the development of an autoimmune response after the first dose with adults. Given that POTS may occur after a viral infection, it may suggest that a longer history of viral exposure in adults may need to be considered as possible priming towards autoimmunity, with the vaccines or COVID acting as the re-exposure incidences.
This may partially explain the results seen here with 2 or 3 doses provides if we argue that children may not be exposed to viruses to the same degree as adults would. Of course, the role of childhood vaccinations in autoantibody formation cannot be discounted as this argument would require an examination of vaccines or childhood infection as being associated with autoimmunity.1
Treatment and Management of SFN/POTS
Given wider recognition of SFN and POTS related to the vaccines, it’s important to consider the treatment options outlined in these case reports. Note that, for the most part, the approach taken by many clinicians were to provide patients with medications that would manage symptoms, and not cure the patients.
For instance, beta-blockers were provided to POTS patients to help with tachycardia, as well as Gabapentin for some SFN patients to manage the tingling and numbness in extremities. Ivabradine also appeared to be used in a few cases, in particular for patients who were noted to have sinus tachycardia in particular.
Based on the details provided, especially with respect to SFN, it appears that resolution of symptoms comes with regrowth and formation of new neurons to compensate for the ones lost due to neuropathy as seen in the report from Mastropaolo, M., & Hasbani, M. J.
This may explain the heterogeneity in symptom resolution for patients wherein some may see fast neurite formation and quicker symptom resolution while others may take far longer. This may also explain the resolution of POTS in some of these case reports as well.
Note that this notion of nerve regeneration is complex, controversial2, and may take a very long time. Even in the Mastropaolo, M., & Hasbani, M. J. case report the skin biopsies taken from the calves showed that more than 250 days lapsed between each biopsy, with IVIG treatment started in between the biopsies. Recovery may have occurred sooner, but still points to a a long process.
If autoimmunity is considered, it’s important to take into account persistence of autoantibodies as well when considering the resolution of symptoms. This raises a question of whether autoantibody persistence may be due to antigen persistence. In any given case, the persistence of the underlying cause of the neuropathy would suggest that removal of these factors (whether antigen or antibody), would be necessary for treatment.
Thus, this leads us to the interesting use of IVIG and plasma exchange in the case reports noted above.
Intravenous Immunoglobulin3 (IVIG) is a rather interesting treatment as it has been used for both immunodeficiency as well as autoimmunity. IVIG is a collection of antibodies from many healthy individuals, sometimes within the hundreds or thousands of donors. The use of such treatment comes with the intent of flooding the body with antibodies. In those who are immunodeficient, some of these antibodies may show neutralizing capacities and may help fight off infections (usually defined as a low-dose of IVIG). At a high-dose, the antibodies may modulate immune responses and even inhibit the autoantibodies, leading to a reduced autoimmune response as well as reduced inflammation.
Therapeutic plasma exchange is more straightforward. With TPE plasma is separated from the red blood cells of a patient and replaced with a serum, usually one that contains albumin in place of the plasma. The intent of this treatment is to remove the underlying causes of autoimmunity, either by way of removing autoantibodies, antigens, inflammatory markers, or various cell complexes.
Zanatta, et al.4 provides a more in-depth explanation for the use of TPE:
TPE appears to be effective for some forms of autoimmune-related neurological diseases.5
Given the Schelke, et al. case report, it raises a question of whether plasma exchange may be a treatment option worth considering for some Long COVID haulers or vaccine-injured individuals.
Remember that plasma exchange does not discredit the possibility of the spike effect of adverse reactions. However, it’s important to remember that, irrespective of what we think of the vaccines, they are still producing anti-spike antibodies. One would surmise that repeat exposure to the spike antigen should be met with neutralization, and so adverse events following any subsequent dosing should be considered under the reduced spike/increased antibody paradigm.
But more importantly, all of this raises a critical thing to consider. If autoimmunity is a big driver of these adverse reactions, it would suggest that each dose of the vaccine, by way of alerting the body to unintentionally attack itself, would be extremely harmful to the individual.
Consider that exposure to an allergen, such as a peanut, for the first time may be met with discomfort with an itchy throat, lips, and other symptoms such as that. The first exposure, a priming moment, may not produce a severe overreaction of the immune system. However, a re-exposure to that same allergen may be deadly- the body would be ready to target the allergen a second time, and in this case the second exposure may lead to anaphylaxis.
Thus, any subsequent dose of a vaccine, if activating autoantibodies, may be more harmful than the preceding doses. It would also suggest that there is no true “safe” dosage if any repeat exposure would eventually lead the body down the path of autoimmunity.
There’s more to be said in particular to POTS and SFN when it comes to autoimmunity, but I’ll save that for another post and refer people to an older autoimmunity post in the meantime.
One thing I will end this post on is something that has yet to be answered, which is why exactly a 2nd dose was ever needed, or was given so close to the 1st dose. Pfizer/BioNtech was recommended to be give 3 weeks after the 1st dose, with Moderna recommending a 4-week wait.
consider in the above post the concept of epitope spreading, whereby prolonged exposure to an antigen provides off-site epitope recognition which may increase the risk of autoimmune disease. In the same ways that there is an argument of prolonged spike exposure occurring with such a short timeframe between doses, the same can be said about the possible increased risk of autoimmunity occurring.
More importantly, if we are concerned about the failures in these 2-dose clinical trials, shouldn’t we levy the same criticisms against initial studies on 1-dose vaccine effectiveness, which were used to argue why 2-doses were better?
In a very cynical sense, I am curious if all of this was rigged from the start, where concept of 2-doses being “fully vaccinated” was a gaming of public health right that likely contributed to all of these adverse reactions.
Substack is my main source of income and all support helps to support me in my daily life. If you enjoyed this post and other works please consider supporting me through a paid Substack subscription or through my Ko-fi. Any bit helps, and it encourages independent creators and journalists such as myself to provide work outside of the mainstream narrative.

One hypothesis may suggest that the discrepancy in innate and adaptive immunity related to age may actually be an adaptation to prevent autoimmunity early on in life. I haven’t explored this idea yet but it would be an interesting one to consider.
I state controversial due to the fact that certain neurons don’t appear to regenerate, and so I hesitate to make broad remarks on the regeneration capacity of neurons.
Arumugham VB, Rayi A. Intravenous Immunoglobulin (IVIG) [Updated 2022 Jul 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK554446/
Zanatta, E., Cozzi, M., Marson, P., & Cozzi, F. (2019). The role of plasma exchange in the management of autoimmune disorders. British journal of haematology, 186(2), 207–219. https://doi.org/10.1111/bjh.15903
Nieto-Aristizábal, I., Vivas, Á. J., Ruiz-Montaño, P., Aragón, C. C., Posso-Osorio, I., Quiñones, J., Rivillas, J. A., & Tobón, G. J. (2020). Therapeutic Plasma Exchange as a Treatment for Autoimmune Neurological Disease. Autoimmune diseases, 2020, 3484659. https://doi.org/10.1155/2020/3484659





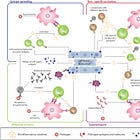
Epitope spreading and cross reactivity...similar, but different?
2ND SMARTEST GUY IN THE WORLD SUBSTACK
NEW - EU Digital Commissioner is Threatening to Shut Down Social Media Platforms in the Event of Social Unrest
We have only just begun...
https://www.2ndsmartestguyintheworld.com/p/new-eu-digital-commissioner-is-threatening
ARCHIVED ⬇️
https://archive.md/2023.07.13-134902/https://www.2ndsmartestguyintheworld.com/p/new-eu-digital-commissioner-is-threatening