Growing evidence suggests an autoimmune response post-COVID infection and COVID vaccination.
A very broad, cursory glance at some mounting evidence suggesting widespread autoimmunity which may explain some of the symptoms of Long COVID and adverse reactions seen.
Growing empirical evidence has continued to suggest that both a SARS-COV2 infection, as well as COVID vaccination, may be related to various post-viral syndromes (i.e. Long COVID) in the form of autoimmune disease.
I have been meaning to cover this topic a while back, but had shelved a deeper look into the literature because of the amount of research needed and time commitment.
However, in several prior posts I made remarks that some of the adverse reactions seen may be attributed to autoimmunity in some regard, as several people have considered that myocarditis may be associated with an autoimmune response that targets the heart. More recently covered, some people who have received COVID vaccines appear to have a developed N-methyl-d-aspartate receptor antibody-mediated encephalitis (NMDAR-E).
The idea of autoimmunity related to viral infections, and in particular SARS-COV2 is actually not new. Some of the evidence of such an autoimmune response after a severe COVID infection can even date back to the early months of the pandemic.1
However, in recent months more reports have come out adding further evidence towards an autoimmune response.
We’ll take a rather broad look at some of the information and hopefully save it for an anthology series. So this information will be extremely broad and not fully researched.
What is autoimmunity?
The term autoimmunity has been thrown out quite a lot, and in may cases discussions may make it appear as a disease without a cause (as in it appears to just happen).
As the name implies (auto inferring self) autoimmunity refers to a phenomenon in which the immune system mounts a response to endogenous structures such as proteins, cells, and tissues, usually causing destruction of these cells.
Various factors are related to autoimmunity, including autoreactivity in which both B and T-cells react and target the body’s own cells. Some of this action may be dictated by autoantibodies, in which some of the antibodies our bodies produce are able to bind or stick to our own cells and target them for destruction.
In consideration of stickiness, keep in mind that antibodies mainly differ by the amino acid sequence that makes up their paratope. In several cases some people may unfortunately luck out and produce antibodies that don’t just stick to endogenous substances, but also to proteins and enzymes belonging to the body.
What’s important to remember is that our immune response is highly tailored, but it is not completely selective in their targets.
At the end of the day, we are all made up of proteins, and most cellular processes are dictated by how proteins or other compounds interact with one another, usually through electrostatic interactions such as hydrogen bonding, ionic bonding, or Van der Waals forces.
These interactions themselves may have preferences (opposites attract, for example), but that doesn’t mean that other interactions may not occur. For instance, even if a positive charge attracts towards a negative charge you can’t dictate which positive charges will interact with negative charges (in a very general sense). Essentially, our body is constantly teeming with proteins and molecules that may just be sticking to one another as they move.
This is why heavy metal toxicity can be derived from competitive binding between heavy metals and key ions ions2, or why some chemicals act as endocrine disruptors by sharing similar structures to our hormones, with one recent example being dioxin.
And so because chemical reactions are heavily dependent on the “sticking” capacity between molecules it’s not too much of a surprise to consider that, for some reason, our immune system and antibodies may stick to some of our own proteins and cells.
But that’s a rather broad generalization.
Aside from autoantibodies it may be the case that antigen presentation may selectively pick up epitopes that are analogous in peptide sequence to those related to our own proteins.
One well known example is celiac disease. In this case, people who carry the human leukocyte antigen (HLA) alleles for either DQB1:02 or DQB1:08 (either heterozygous or homozygous for either) may be more at risk of developing CD. These alleles are part of the MHC Class II aspect of HLA, which are antigens that bind exogenous epitopes and are located on the surface of antigen-presenting cells. In this case, people who are carriers of these alleles have antigen-presenting cells that are more prone to binding to deamidated gliadin broken down from gluten. This response may lead to targeting of the body’s own enzymes called transglutaminases, which leads to inflammation and gut permeability.
It shouldn’t come as a surprise that transglutaminases share some sequence similarity to gliadin as it serves as the enzyme that deamidates (removes an amide functional group) gluten. As such, the cross-reactivity of autoantibodies may be due to amino acid homology between the enzyme and gliadin.
Overall, it appears that gluten serves as an environmental sensitizer, leading to an immune response in which deamidated gliadin is recognized by antigen-presenting cells carrying the proper DQB1 haplotype, which THEN causes the body to target transglutaminases due to cross-reactivity.
Some more can be read on celiac disease autoimmunity in a review from Caio, et al.3 What's interesting is that some evidence may suggest cross reactivity towards bacterial peptides as being a primer for celiac disease as well, adding more complexity to the matter.4
This may seem like a bit much for a broad overview, but note that autoimmunity occurs due to multiple dynamics. HLA alleles vary from each individual, and the type of antibodies one makes also differs from one another. This doesn’t even take into account environmental factors as well. It’s important to keep in mind multiple aspects all contribute to one’s risk of developing autoimmunity.
Mechanisms of infection-related autoimmunity
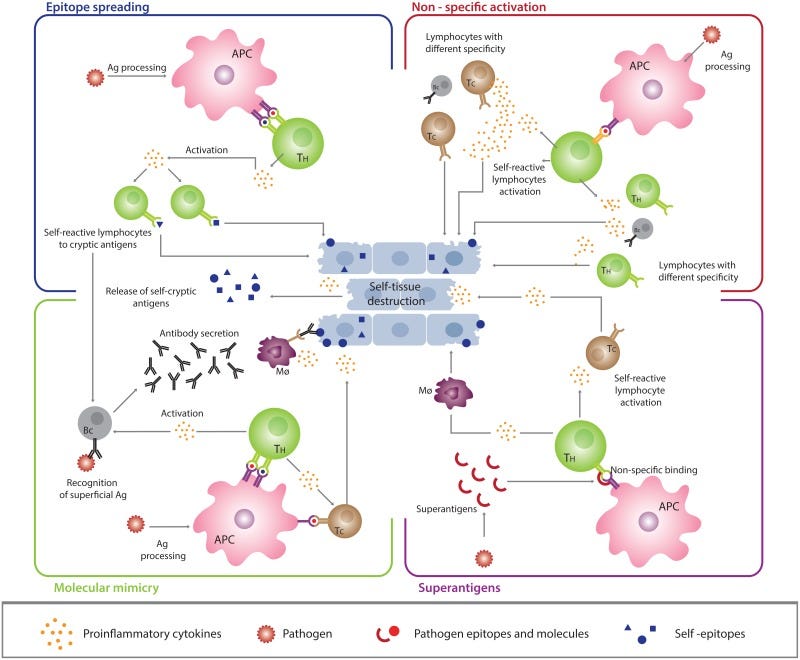
For the most part bacteria and viruses act as environmental agents. This raises the question of how infections from such agents may lead to an autoimmune response. Years of evidence have noted a correlation between infection and vaccination with respect to autoimmunity.
In general, some of these explanations fall along 4 possible models. Below will be a more simplified explanation with additional information being found in the following reviews:
Arango, et al.5: Infection and autoimmune diseases.
Fousteri, G., & Dave Jhatakia, A.6: Viral Infections and Autoimmune Disease: Roles of LCMV in Delineating Mechanisms of Immune Tolerance.
This review refers to the virus lymphocytic choriomeningitis virus (LCMV) when explaining autoimmune disease. LCMV appears to serve as one of the main models for autoimmunity and has been utilized in many animal models to examine this phenomenon.
1. Molecular Mimicry
One possibility is that antigenic epitopes may be similar in sequence to human proteins. This molecular mimicry may lead to production and release of cross-reactive autoantibodies that target both exogenous antigens as well as human proteins, which may then cause destruction of our own cells. A clear example of this is the one shown for celiac disease above. In general, molecular mimicry has been one of the most considered models of autoimmunity, with several decades of research investigating molecular mimicry in animal models.
With respect to molecular mimicry a hypothesis was proposed in 2003 by von Herrath, et al.7 described as the "fertile-ground hypothesis”, in which it was argued that a window of higher risk of autoimmunity may arise, likely after exposure to a microbe or to an inflammatory adjuvant:
Taken together, the above ideas indicate that, as suggested previously64, microbial infection (or even the administration of powerful adjuvants36) can induce a temporary immunological state for which we propose the term 'fertile field'. If this short-lived field is 'sown' with other antigens (environmental, viral or self; the latter will vary depending on the host genotype), it can yield a pathogenic 'harvest' of autoreactive or autoaggressive T cells. This viewpoint clarifies why microorganisms should be seen as factors contributing to, rather than being the sole causative agents of, autoimmune (and allergic) diseases. Organ-specific autoimmune diseases such as T1D and MS are most commonly associated with viruses that replicate extensively in the affected tissue, consistent with the idea that infection induces a transient, and localized, fertile field — 'fertilizing' the growth of organ-specific lymphocytes.

2. Epitope Spreading
The response to an antigen is not even; certain epitopes are more immunodominant (induce a stronger immune response) such as the RBD of the SARS-COV2 spike protein. However, as exposure to an antigen prolongs other epitopes may become recognized and targeted, including ones from other antigens that may be sequestered within the microbe such as the nucleocapsid protein of SARS-COV2. The targeting of these different epitopes may increase the possibility of recognizing a cross-reactive region of an antigen. This hypothesis is termed epitope spreading and has been recognized in some viral models.
Overall, this hypothesis suggests that giving the immune system time to learn all facets of an antigen may come at a cost of recognizing an epitope that is similar to our own proteins and lead to autoimmunity.
3. Bystander Activation
The immune system appears to respond somewhat nonselectively to inflammatory markers. As such, inflammation related to other independent events may still lead to the unfortunate activation of autoreactive T-cells. This is, not surprisingly, termed bystander activation as the cells involved aren’t ones directly involved with dealing with an invading pathogen, but nonetheless become activated by the inflammatory biomarkers released.
An example of this can be found in prior posts in which it was argued that the inflammatory response towards a viral infection may activate gut-related immune cells and cause gut dysbiosis through altering the microbiome and enhancing gut permeability, and may be a cause of secondary bacterial infection. In this case the inflammatory response within the lungs may lead to systemic immune responses.
4. Superantigens
Some antigens may lead to recruitment and activation of many T cells. In this case, these superantigens don’t require antigen processing or presentation, but bind to different sites on the surface of T and B cells which elicit a strong immunostimulatory effect. The end result may be a chronic, robust inflammatory response that may influence autoimmunity.
A more technical review on superantigens can be found in Deacy, et al.8
Note: When reviewing some of the above models note that they aren’t exactly distinct from one another. Like cross-reactive antibodies, many of these models are intertwined, and it’s more than likely that combinations of the above are involved in autoimmunity.
Do all infections lead to autoimmunity?
Given these models one may raise the question as to whether pathogens will inherently induce autoimmunity in those predisposed to the disease.
Fortunately, it appears that may not be the case. In contrast to an “every infection will lead to autoimmunity” fear, some evidence suggests that exposure to microbes along the lines of the hygiene hypothesis may actually be protective against autoimmunity.
Some of this evidence stems from epidemiological data and is confounded by many modern factors. However, the general correlation suggests a higher rate of autoimmunity in the modern age, which may correlate with a larger emphasis on sterility in the past few years especially in the wake of COVID.
The lack of challenging the immune system may be detrimental, as routine exposure to pathogens may serve as a method of controlling and better directing the immune system’s responses towards actual pathogens and not just itself. This form of immune regulation has been suggested in various animal models which juxtapose the suggestion that an infection will inherently lead to autoimmunity.9
Given that sensitization to cross-reactive epitopes may induce autoimmunity, one can also argue that tolerance to antigens may actually suppress an autoimmune response. This may include depletion of cross-reactive B and T-cells, leading to a more tailored immune response that targets non-self epitopes of antigens while sequestering self-antigen responses.
It’s interesting to consider tolerance within the context of SARS-COV2 infection and vaccination. Concerns have been raised over the increase in IgG4 seen in various studies of people who have received boosters, with suggestions that such a phenomenon may lead to tolerance of the spike and possible ambivalence towards future infections.
However, what has yet to be characterized is the makeup of the antibodies that are switching subclasses (i.e. which epitopes they are targeting, and if those antibodies are cross-reactive), and why this response varies among individuals. Given what has been outlined so far, I’m curious whether IgG4 subclass switching may be a corrective response of the immune system to self-antibodies, such that IgG4 subclass switching may be occurring more frequently in those who are prone to an autoimmune response or likely have experienced some form of autoimmunity with respect to the vaccines.
This would suggest a possible genetic basis related to different HLA haplotypes and whether some may be more predisposed to antigen presentation of cross-reactive epitopes. Such a hypothesis may be easily solved by isolating IgG4 antibodies and testing them in vitro for cross reactivity. Observational studies may also consider examining IgG4 antibodies as correlates for adverse reactions such as myocarditis.
In any case, the concept of tolerance is more nuanced than ambivalence to an antigen. It’s one of the reasons why characterizing the antibodies in particular and looking for individual differences may provide a clearer picture of why certain people may be predisposed to autoimmunity or adverse reactions.
Autoimmunity and COVID
As mentioned before autoimmunity has been documented since the first few months of the pandemic. However, within the past few weeks several review articles have come out providing more details towards autoimmunity.
Much to my chagrin, I should note that several of these articles came to my attention by way of Eric Topol’s recent Substack piece.
I have many issues with how Eric Topol interprets studies, and he’s considered one of the premier voices in pushing the vaccine so I won’t say that I have much affection towards him.
Nonetheless, he did collect some articles with respect to autoimmune diseases post-SARS-COV2 infection. Of course, he also simultaneously downplayed the role of autoimmunity with respect to COVID vaccines, barely mentioning evidence of autoimmunity post-vaccination and playing a game of autoimmunity relativism.
But I digress. One source cited by Topol includes the following editorial published in Nature10:
The review references the cited retrospective study published in The Lancet11:
I haven’t examined this study in full, but the study compared outcomes in those who were infected with SARS-COV2 with those who were not. Both groups were not vaccinated.
One forest plot summary notes the various autoimmune diseases that appear to correlate with a COVID infection:

Again, what appears to be missing from Topol’s assessment is the evidence of autoimmunity post-vaccination.
One recent, comprehensive review from Guo, et al.12 lists many of the case reports and case series detailing these autoimmune diseases.
The organization of the review is not conducive for presenting in an aesthetic manner, but the autoimmune diseases recorded include the following:
Immune-mediated nephropathy
IgA Nephropathy
Membranous Nephropathy
Lupus Nephritis
Focal segmental glomerulosclerosis
Autoimmune rheumatic diseases
Rheumatoid arthritis
Antiphospholipid syndrome
Adult-onset Still’s disease
ANCA-associated vasculitis and Giant cellarteritis
Sjogren’s syndrome and Behçet's disease
Autoimmune hepatitis
Type 1 diabetes mellitus
Autoimmune hemolytic anemia
There’s several autoimmune disease that have likely been missed in this review. Also, it’s important to consider a possible incidental nature of some of these autoimmune diseases. However, given the nature of autoimmunity it’s hard to wave off any possible correlation as being purely incidental.
Now, there’s a question as to why these autoimmune diseases are occurring. Topol’s game of autoimmunity relativism tends to obfuscate a mechanistic reason for this response.
A main explanation may be related to the spike protein’s pathogenicity. The spike protein is a shared structure across all vaccine platforms as well as in infections.
In this case, the spike may lead to recruitment of cross-reactive antibodies and the following autoimmune response. The spike has been considered to carry superantigenic epitopes13 which may also contribute to this phenomenon. In both circumstances the highly inflammatory response may also lead to bystander activation.
With respect to the vaccines there would be a plausible concern that the addition of adjuvants may play a critical contribution in bystander activation.
A broader examination will likely be saved for a future post. However, what remains to be answered is the course of an infection relative to vaccination that may lead to the autoimmune diseases seen.
But in any given case the evidence here raises even further emphasis on the need to examine individual differences in relation to autoimmune disease from either an infection or vaccine. Lack of comprehensive evidence suggests that we can only view these case reports from a superficial position.
But there’s also a greater need to not be so quick to downplay adverse reactions or the possibility of Long COVID. There have been several arguments made that have attempted to either downplay myocarditis rates from a COVID infection or to downplay the events of Long COVID. Consider here that autoimmune disease itself is likely to be an indication of Long COVID, and thus both those who have been infected and those who have been vaccinated are at risk of developing Long COVID in some capacity.
This is why the game of relativism is a game both teams will lose at if it means obfuscating the mechanistic aspects of why autoimmunity or Long COVID is occurring.
A longer discussion will be saved for a future date as I’ve intended to cover autoimmunity as an anthology series. As a forewarning, I may be out for the next few days, and if so I will make a future post making that notice.
Substack is my main source of income and all support helps to support me in my daily life. If you enjoyed this post and other works please consider supporting me through a paid Substack subscription or through my Ko-fi. Any bit helps, and it encourages independent creators and journalists such as myself to provide work outside of the mainstream narrative.

Galeotti, C., Bayry, J. Autoimmune and inflammatory diseases following COVID-19. Nat Rev Rheumatol 16, 413–414 (2020). https://doi.org/10.1038/s41584-020-0448-7
Witkowska, D., Słowik, J., & Chilicka, K. (2021). Heavy Metals and Human Health: Possible Exposure Pathways and the Competition for Protein Binding Sites. Molecules (Basel, Switzerland), 26(19), 6060. https://doi.org/10.3390/molecules26196060
Caio, G., Volta, U., Sapone, A., Leffler, D. A., De Giorgio, R., Catassi, C., & Fasano, A. (2019). Celiac disease: a comprehensive current review. BMC medicine, 17(1), 142. https://doi.org/10.1186/s12916-019-1380-z
Petersen, J., Ciacchi, L., Tran, M.T. et al. T cell receptor cross-reactivity between gliadin and bacterial peptides in celiac disease. Nat Struct Mol Biol 27, 49–61 (2020). https://doi.org/10.1038/s41594-019-0353-4
Arango MT, Shoenfeld Y, Cervera R, et al. Infection and autoimmune diseases. In: Anaya JM, Shoenfeld Y, Rojas-Villarraga A, et al., editors. Autoimmunity: From Bench to Bedside [Internet]. Bogota (Colombia): El Rosario University Press; 2013 Jul 18. Chapter 19. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459437/
Fousteri, G., & Dave Jhatakia, A. (2019). Viral Infections and Autoimmune Disease: Roles of LCMV in Delineating Mechanisms of Immune Tolerance. Viruses, 11(10), 885. https://doi.org/10.3390/v11100885
von Herrath, M., Fujinami, R. & Whitton, J. Microorganisms and autoimmunity: making the barren field fertile?. Nat Rev Microbiol 1, 151–157 (2003). https://doi.org/10.1038/nrmicro754
Deacy, A. M., Gan, S. K., & Derrick, J. P. (2021). Superantigen Recognition and Interactions: Functions, Mechanisms and Applications. Frontiers in immunology, 12, 731845. https://doi.org/10.3389/fimmu.2021.731845
Smatti, M. K., Cyprian, F. S., Nasrallah, G. K., Al Thani, A. A., Almishal, R. O., & Yassine, H. M. (2019). Viruses and Autoimmunity: A Review on the Potential Interaction and Molecular Mechanisms. Viruses, 11(8), 762. https://doi.org/10.3390/v11080762
Sharma, C., Bayry, J. High risk of autoimmune diseases after COVID-19. Nat Rev Rheumatol (2023). https://doi.org/10.1038/s41584-023-00964-y
Chang, R., Yen-Ting Chen, T., Wang, S. I., Hung, Y. M., Chen, H. Y., & Wei, C. J. (2023). Risk of autoimmune diseases in patients with COVID-19: A retrospective cohort study. EClinicalMedicine, 56, 101783. https://doi.org/10.1016/j.eclinm.2022.101783
Guo, M., Liu, X., Chen, X., & Li, Q. (2023). Insights into new-onset autoimmune diseases after COVID-19 vaccination. Autoimmunity reviews, 103340. Advance online publication. https://doi.org/10.1016/j.autrev.2023.103340
Brown, M., & Bhardwaj, N. (2021). Super(antigen) target for SARS-CoV-2. Nature reviews. Immunology, 21(2), 72. https://doi.org/10.1038/s41577-021-00502-5





I wrote a literature review last July. I thought it would take a couple of hours. Instead it took days and was extremely concerning. Unfortunately we are starting to see the consequences now.
Be aware this will take years to play out, autoimmunity can have a long latency.
Autoimmune disorders: COVID-19, spike protein & homologous epitopes
https://doorlesscarp953.substack.com/p/autoimmune-disorders-covid-19-spike
Thank you for the very interesting and thorough article. Dr. McMillan has also been beating the drum about autoimmunity issues since the beginning of the pandemic. I think you both are absolutely on the right track.
https://philipmcmillan.substack.com/p/health-risks-associated-with-the?
These COVID-19 researchers have been focused on understanding how the spike protein interacts with the immune system.
Research seems to indicate that exposure to spike protein has a number of long term health consequences that need to be delineated.
Here is a recent paper:
Gerlach, Joachim, et al. "The immune paradox of SARS‐CoV‐2: Lymphocytopenia and autoimmunity evoking features in COVID‐19 and possible treatment modalities." Reviews in Medical Virology (2023): e2423.
https://philipmcmillan.substack.com/p/the-silent-covid-vaccine-death?
In the context of covid vaccine silent organ damage, my research has pointed to low level autoimmunity around ACE-2 and a number of other proteins as the critical target.
The standout other protein is neuropilin-1.