Viral infections, the microbiome, and secondary bacterial infections
An overview of how viral infections can readily lead to dysbiosis of the microbiome, and how the end result of bacterial co-infections may not be too uncommon.
Edit 1/3/2023: This post originally did not include a citation for the Hanada, et al. review. A citation has been included under Footnote #7.
This post is one of a series of posts that will dive deeper into the microbiome. The prior one broadly discussed how the attempts at sterility likely had detrimental effects on the microbes that live in and on us.
In this post, we’ll take a look at viral infections and their interesting influence on the microbiome.
Note that this review, like others, can’t provide a thorough overview of this topic and serves as a starting point based on some of the information I was able to find. Please refer to the articles referenced in this and other posts for additional information.
The title of this post would seem like a rather nonsensical association. It wouldn’t quite make sense to immediately assume that getting the flu should somehow influence your gut bacteria, or make you more likely to get infected by another bacteria.
However, evidence suggests that several viral infections have been associated with follow-up, secondary bacterial infections.
For instance, the 1918 flu outbreak has been partially blamed on bacterial infection, as bacterial pneumonia has been credited as being one of the main causes of death for many people1. In this case, it would appear that bacterial infections were secondary to viral infections, and the authors note that both a viral and bacterial infection may have been what led to worse outcomes compared to a viral infection alone.
Indeed, bacterial co-infection appears to far more common than one would realize, and in the era of COVID it’s been argued that many cases of acute respiratory distress syndrome (ARDS) and pneumonia may be owed to bacterial infections, rather than SARS-COV2 itself2-we'll examine this in a separate post.
This idea has, of course, raised questions and skepticism as to whether such pandemic numbers are actually accurate, or if deaths from these events may be improperly blamed on the wrong pathogen.
These arguments tend to examine the virus/bacteria paradigm as being an either/or scenario, in that someone must have either died from a viral infection, or died from a bacterial infection, without addressing underlying dynamics that may make co-infection with both a likely scenario.
In reality, both viral and bacterial infections may go hand-in-hand, in that viral infections may make one more susceptible to bacterial infections.
One key factor in this phenomenon is the gut microbiome, and mounting evidence suggests that a viral infection may alter the gut microbiome, and thus lay the groundwork that allows for secondary bacterial infections.
The Gut-Lung Axis
Again, upon first glance it may seem strange to consider that the gut may play a role in respiratory infections. However, the bacteria that reside within our gut (as well as in other regions of our body) seem to have several immunomodulatory effects, provide physical barriers from other bacteria as well as viruses, and may provide us with necessary nutrients to fight off infections.
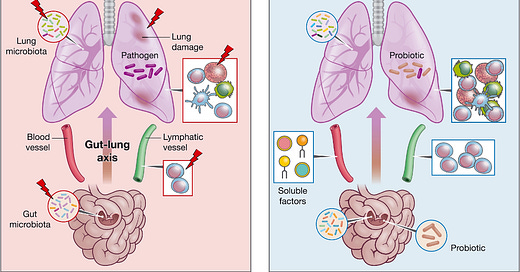
The crosstalk between what goes on in the lungs and what goes on in the gut is becoming more critically assessed, and may provide some key insights into what goes on during a viral infection.
This dynamic, aptly-termed the gut-lung axis, illustrates the significant interactions between the gut and the lung (and vice versa), and how these interactions either benefit or harm an individual (Enaud, et al.3).
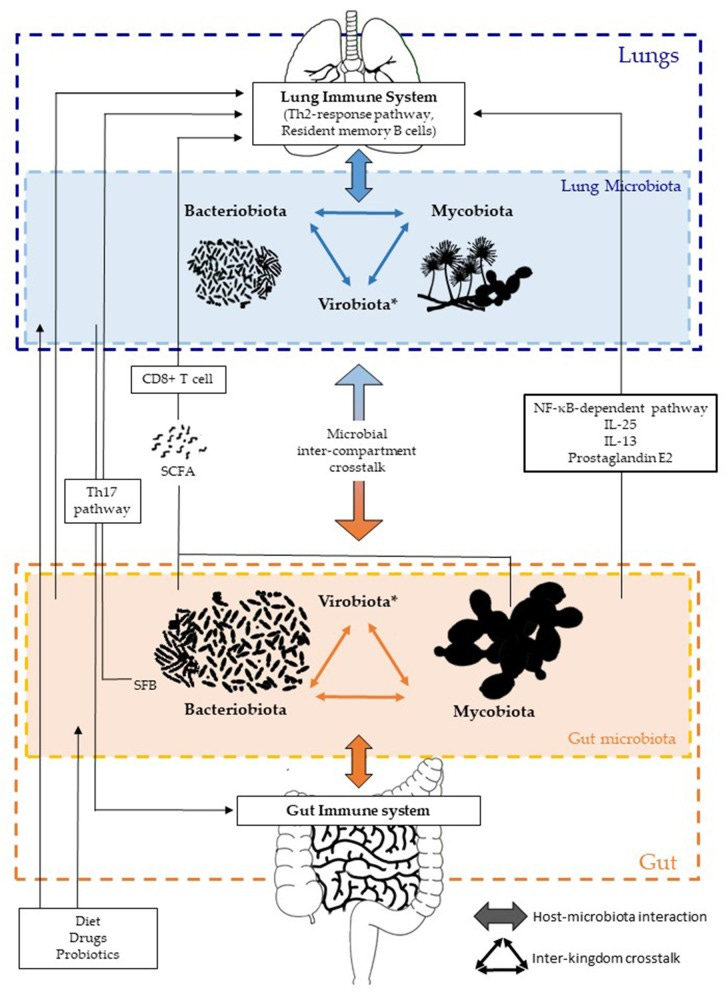
It’s part of a growing theory called the holobiont theory4, which looks at the roles of a host’s microorganisms/microbiome as it relates to that host’s overall health. Even now, some research are examining the benefits of fungi that reside inside of us, termed the mycobiota, and growing evidence suggests that our lack of parasites may be one reason for high allergy rates in the WEIRD world.
As such, the ever-changing microenvironment during an infection has a greater influence on disease severity and secondary infections than as once thought.
This discussion will generally look at how the dynamics between the gut microbiome may influence respiratory infections5, and how respiratory infections may lead to downstream alterations in the gut microbiome.
But first, we’ll discuss briefly how the microbiome actually contributes to creating an inhospitable environment for viruses and bacteria alike.
Antimicrobial mechanisms of the microbiome
The microbiome plays a critical role in protecting the host through various mechanisms. Therefore, it wouldn’t come as a surprise to consider that failures in maintaining a microbiome (dysbiosis) may increase the risk of infection.
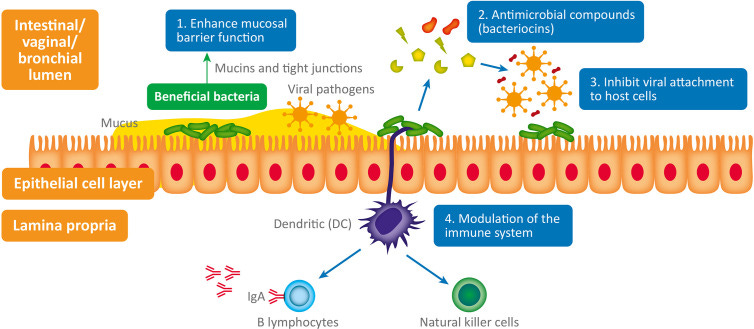
A review from Harper, et al.6 outlines these possible protective mechanisms in the figure below, along with this paragraph:
The interplay between bacteria, viruses and host physiology is complex, and we still have much to learn. Despite this, a mounting body of evidence is beginning to reveal the fascinating contribution of both commensal and probiotic organisms to host defense against viral pathogens (Li N et al., 2019). When virus are exposed to mucosal surfaces (e.g., vaginal, respiratory, or GI) they have three broad lines of defense to overcome: the mucus layer, innate immune defenses and adaptive immune defenses (Kumamoto and Iwasaki, 2012). Evidence suggests that various commensal and probiotic bacteria influence each of these lines of defense with important relevance to a range of viral infections. The antiviral mechanisms responsible (summarized in Figure 1) are both direct and indirect and include: 1) Enhanced mucosal barrier function (Lieleg et al., 2012; Schroeder, 2019), 2) Secretion of antiviral antimicrobial peptides (AMPs) (Torres et al., 2013; Quintana et al., 2014); bacteriocins, 3) Inhibition of viral attachment to host cells (Botić et al., 2007; Su et al., 2013), 4) Modulation of antiviral innate and adaptive leucocyte function (De Vrese et al., 2005; Jounai et al., 2012).
Hanada, et al.7 elaborates further by highlighting the benefits of short-chain fatty acids (SCFAs) produced by gut bacteria and their effects on neighboring host cells, as well as different innate and adaptive immune cells8:
First, bacterial metabolites generated by gut commensals contribute to the maintenance of intact epithelial integrity, regulatory T-cell development, and a relatively anti-inflammatory immune state. In particular, short-chain fatty acids (SCFAs) such as acetate, propionate, and butyrate are fermentation products of dietary fiber and carbohydrates by large intestinal bacteria (30). In addition to being a major energy source for intestinal epithelial cells, SCFAs promote the development of naive CD4+ T cells into regulatory T cells (31, 32), induce “tolerogenic” dendritic cells in the intestinal mucosa (33), and limit autoimmity (34, 35). At the same time, microbial metabolites are integral for promoting immune responses in the gut against pathogens, including inducing secretion of IL-18 (36) and defensins (37, 38). Thus, the products of microbiome metabolism are integral to the appropriate regulation of mucosal barrier integrity and immune homeostasis. In addition, specific members of the bacterial community have been shown to foster the proper maturation and development of the immune system. While this is still an area undergoing intense investigation, one notable example is the discovery that segmented filamentous bacteria are critical promoters of intestinal mucosal IgA production (39, 40)and Th17 cell induction (41, 42).
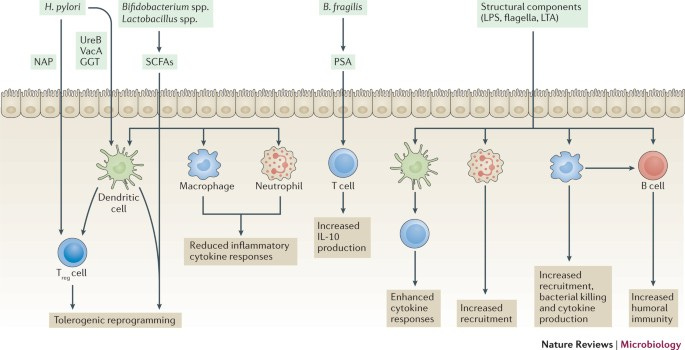
And another review from Li, et al.9 notes some other antiviral mechanisms from commensal bacteria, with corresponding studies listed:

Although the microbiome is critical to our overall health, some findings suggest that the microbiome may actually aid in the pathogenicity of some viruses, again noted in Li, et al.’s review, with a good deal of research focusing on the poliovirus:

It’s fascinating that several of these studies, conducted in mice, deployed antibiotic treatment10 and found reduced viral infectivity in treated mice when compared to untreated mice, as appears to be the case with poliovirus studies.
Part of this explanation may lie in the immunomodulatory mechanisms of the microbiome, which may prevent a strong immune response needed to eliminate specific viruses. This may run counter to other viral infections, in which a hyper-inflammatory response may actually end up harming the body.
This should highlight the fact that the microbiome itself is extremely complex, and that some viruses may utilize our microbiome for their own benefit. It’s also critical to understand that the microbiome/pathogen paradigm should also be seen as a balancing act.
With that being said, it’s clear that the microbiome is critical in protecting us from other, more harmful pathogens.
It makes sense, then, to suggest that dysbiosis may be related to worse outcomes from viral infections, as a disruption to the microbiome homeostasis may alter immune function.11
Note that gut dysbiosis has been implicated in many diseases such as obesity, autoimmune disease, asthma, diabetes, and chronic viral infection, all of which are related to immune dysfunction and increased risk of severe infection.
And so it’s important to note that the gut-lung axis is bidirectional, in that a healthy gut may be conducive to a healthy lung and protection from pathogens.
However, a poor lung may strangely also lead to a poor gut.
viral infections and the microbiome

Now that we’ve covered how the microbiome can protect us from infection, let’s look at what happens when a respiratory viral infection takes place.
More importantly, we’ll see what dynamics may occur that may lead to secondary bacterial infections.
In that regard, it’s not too far-fetched to assume that effects which harm the microbiome will inherently make one more predisposed to infections of other sorts.
Interestingly, evidence suggests that viral infections have influences on the microbiome, as noted by Li, et al.:
While modulation of the commensal microbiota by viruses is still poorly understood, studies to date do suggest an important role of virus infection in inducing microbiota dysbiosis. This is true for HIV/SIV infection, influenza virus infection, HBV or hepatitis C virus (HCV) infection and norovirus infection, as discussed in detail below. In addition to these four types of viral infections, alteration of the gut microbiota following infection has also been described in cases of rotavirus infection in pigs or calves (90, 91), avian leukosis viruses in chickens (92), canine distemper virus infection in giant pandas (93), and white spot syndrome virus infection in crabs (94), although the relevant reports are sporadic and the corresponding mechanistic evidence is very limited.
It’s important to note that many of these studies used animal models, and therefore the difference in gut diversity should be taken into account, as well as the corresponding virus being investigated.
Now, as we consider what may lead to this dysbiosis, a few thoughts may immediately come to mind:
1. A virus may directly target the microenvironment
A viral infection may lead to damaged cells and tissue. This may have direct effects on the microbiome by producing an inhospitable environment for beneficial bacteria, leading to microbiota alterations and increased susceptibility to pathogenic bacteria as well as pathobionts taking over.
A review from Bakaletz, L. O.12 notes the following:
Much of what we have learned to date has been gleaned from animal models which have shown that complex molecular mechanisms underlie the ability of viruses to predispose to bacterial superinfection (see Table 1 ). […] Briefly however, as a general outcome, viral infection can induce destruction of the airway both histologically and functionally. Depending on the virus, the histopathology induced can be relatively mild or severe and include evidence of cell loss, goblet cell hyperplasia, altered mucus secretion and/or biochemistry, disruption of surfactant, reduced ciliary beat frequency, dis-coordinated mucociliary clearance function and reduced oxygen exchange [23]. Each of these effects has long been associated with potential mechanisms by which viruses predispose the respiratory tract to bacterial superinfection.
One of the prevailing notions for SARS-COV2 and gut dysbiosis suggests that the virus may directly bind to ACE-II receptors of the gut epithelial, and this action itself may damage the microenvironment, reduce biodiversity, and lead to leaky gut and diarrhea. This may explain why gastrointestinal issues are considered a common feature of SARS-COV2 infection, although this effect may also be related to downstream alterations to the gut microbiome, which itself may be a cause for GI problems.
Overall, direct damage to the microenvironment of the lungs or gut can have several effects, as the loss of a protective microbiome means that pathogenic bacteria may take over this newly empty space and flourish. This, paired with damaged tissue and cellular function may suggest that the body cannot properly protect from the seeding of pathogens, and thus secondary bacterial infection occurs.
2. A virus may indirectly alter the microbiome
More likely is the suggestion that a viral infection may have an indirect effect on the microbiome, usually through the inflammatory response that alters biodiversity. In this case, the localized targeting of a virus may have systemic effects, including damage and alterations to the gut and other microbiomes.
One example of this appears to come from antiviral, pro-inflammatory molecules such as interferons. Although great for targeting viruses, the activation and release of interferons appear to reduce the level of anaerobic bacteria within the gut and alter the gut homeostasis.
Hanada, et al. notes a few studies suggesting that inflammatory cytokines are related to gut microbiome alterations13 (emphasis mine):
Interestingly, although SFB [segmented, filamentous bacteria] have previously been shown to induce Th17 cells (41, 48), flu-infected mice had increased IL-17A levels and numbers of Th17 cells in the small intestine and colon, which appeared to contribute to intestinal injury (43). In this study, antibiotic treatment prior to influenza infection ameliorated the degree of intestinal injury, but not lung injury, suggesting that gut dysbiosis contributed to local but not systemic inflammation. Other groups have similarly reported increased Proteobacteria (the phylum of which Enterobacteriaceae are members) (44, 45), decreased Firmicutes (which include SFB, Lactobacillus and Lactococcus species), and increased Bacteroidetes (47) following infection by flu or RSVs but not after administration of live attenuated influenza vaccine (LAIV), indicating that live viral infection is required for these changes (47). The increase in Proteobacteria appears to be mediated by type I interferons (IFNs) (18), which not only depleted anaerobic bacteria but also increased susceptibility to secondary Salmonella colitis. […] It has also been shown that influenza infection alters intestinal microbiota composition through type II IFN produced by lung-derived T cells recruited to the intestine (43). Thus, changes in the gut microbiome appear to result not from direct viral effects but from systemic inflammatory signals that travel from the lung and trigger local inflammatory responses in the gut (Figure 1).
And reiterated in Bakaletz, L. O.’s review:
As mentioned above, virus infection induced dysregulation of the proinflammatory cytokine response, including but not limited to that induced by influenza virus, is generally believed to play a major role in predisposing to secondary bacterial infection [3 ,37,41,42]. Whereas type I IFNs have well-characterized antiviral and immunostimulatory properties, when IFN production is mistimed, inappropriate and/or excessive, there can be detrimental effects. Key observations include the role of IFNs in promotion of production of specific cytokines such as immunosuppressive IL-10 and pro-inflammatory IL-6, suppression of cytokines key to the linkage between the innate and adaptive immune response including IL17 and IL-23; reduced function of dendritic cells, macrophages, natural killer cells, CD4+ and CD8+ T-cells, all of which leads to impaired ability to effectively eradicate bacterial co-pathogens [1,37,41,42].
Although information is rather limited, it appears that the inflammatory response may be a key component in perturbing the microenvironment and alter the biodiversity. Inflammation tends to be the center of all maladies, and so it’s not too much of a surprise to consider that inflammatory cascades may affect other parts of the body.
This effect, at least as it relates to interferons, is rather interesting given that several gut microbes are key players in the release of interferons, and so one has to wonder why gut microbes may be responsible for activating the same pathways that may lead to their own death.
In any case, given the fact that the gut microbiome serves as a necessary immunomodulator, it’s quite possible that viruses have evolved a way to target these microbes through activation of inflammatory pathways, which ironically would reduce innate and adaptive immune function, as the key bacteria that serve as immune modulators shift in density.
This may also explain why disturbances to the gut microbiome may have effects on bacterial lung co-infections, as immune responses as a whole may be damaged. This is another example of a disruption to the lung-gut axis and altered homeostasis, and another indication of how complex all of these interactions are.
Another indirect effect of a viral infection could also be nutritional alterations. As noted in previously cited Sencio, et al.14 study, a viral infection may lead one to stop eating. This lack of nutrition may reduce the production of SCFAs, which themselves may attenuate innate and adaptive immune function.
And so starving a cold may be an old wives’ tale, since it may deprive the body of necessary nutrients to construct the SCFAs needed to boost the immune system. Of course, if many of these beneficial bacteria are also on the decline, then this effect may be made moot.
Overall, much of the evidence tends to point towards a more indirect effect of a viral infection on the microbiome, although it’s likely both work to influence secondary bacterial infection. There are several other indirect factors worth considering, however more detailed assessments would be beyond the scope of this article.
viral infection (possibly) begets bacterial infection
Emerging evidence suggests that a respiratory infection may lead to dysbiosis and disturbances to the gut-lung axis. This disturbance may make it more easy for pathogenic bacteria to take hold, taking advantage of the damaged terrain of the lungs and gut, and make it possible for bacteria to infect a host already dealing with a viral infection.
Although this doesn’t occur in all cases, co-infection may be more common than first thought, and may be a key component to severe illness and death from various viral respiratory infections.
However, it’s important to remember that studies that look at these co-infections not only rely on animal models, but may also be coincidental reports. Post-mortem studies may show infiltration of bacteria in those suspected to have died from a viral infection, but this window into the body postmortem may not show the actual progression of a bacterial infection.
The Morens, et al. study of the 1918 flu outbreak relied on postmortem tissues, although antemortem (before death) blood samples showed the presence of various cocci species add some additional context to the bacterial pneumonia argument.
Nonetheless, it’s hard to argue to what extent deaths from viral outbreaks should be contributed to a viral infection, a bacterial infection, or both concurrently, but what accumulating information does tell us is that this may not just be quite common, but may be expected, especially in severe infections and hospitalizations.
With that, apologies for those who were looking for a more robust analysis. The field is fairly new, and much of the research into this dynamic of viral infections and the microbiome are all in their early stages. However, I do encourage readers to look at the citations provided in this article (found in the footnotes below) for some more information.
Now, one thing that hasn’t been discussed yet is where exactly these pathogenic bacteria are coming from. We may assume that many of these infections come from the environment, and in most situations this is likely to be the case.
However, we’ve also mentioned that several bacterial strains within our microbiome are pathobionts; becoming pathogenic when certain criteria are met to allow such an event to occur.
To that, evidence suggests that some of these pathobionts may actually turn pathogenic during a viral infection.
Thus, the deadly bacteria may have been inside of us all along…
Alright, a bit dramatic, but the next post will look at a few case studies in which some bacteria have been recorded to translocate, and we’ll take a look at this scenario as it relates to SARS-COV2 and ARDS.
If you enjoyed this post and other works please consider supporting me through a paid Substack subscription or through my Ko-fi. Any bit helps, and it encourages independent creators and journalists outside the mainstream.

Morens, D. M., Taubenberger, J. K., & Fauci, A. S. (2008). Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. The Journal of infectious diseases, 198(7), 962–970. https://doi.org/10.1086/591708
Farrell, J. M., Zhao, C. Y., Tarquinio, K. M., & Brown, S. P. (2021). Causes and Consequences of COVID-19-Associated Bacterial Infections. Frontiers in microbiology, 12, 682571. https://doi.org/10.3389/fmicb.2021.682571
Enaud, R., Prevel, R., Ciarlo, E., Beaufils, F., Wieërs, G., Guery, B., & Delhaes, L. (2020). The Gut-Lung Axis in Health and Respiratory Diseases: A Place for Inter-Organ and Inter-Kingdom Crosstalks. Frontiers in cellular and infection microbiology, 10, 9. https://doi.org/10.3389/fcimb.2020.00009
Simon, J. C., Marchesi, J. R., Mougel, C., & Selosse, M. A. (2019). Host-microbiota interactions: from holobiont theory to analysis. Microbiome, 7(1), 5. https://doi.org/10.1186/s40168-019-0619-4
Most research have, unfortunately, only dived deeply into the gut and not other microbiomes. In recent years the lung microbiome has been an area of interest, and we may find that microbes live in places that we may not have normally considered. However, because not much is known the information here will focus mostly on the gut microbiome, although the lung may be mentioned at times. It’s important to remember that even with all of the information coming out there’s still a ton yet to be found.
Harper, A., Vijayakumar, V., Ouwehand, A. C., Ter Haar, J., Obis, D., Espadaler, J., Binda, S., Desiraju, S., & Day, R. (2021). Viral Infections, the Microbiome, and Probiotics. Frontiers in cellular and infection microbiology, 10, 596166. https://doi.org/10.3389/fcimb.2020.596166
Hanada, S., Pirzadeh, M., Carver, K. Y., & Deng, J. C. (2018). Respiratory Viral Infection-Induced Microbiome Alterations and Secondary Bacterial Pneumonia. Frontiers in immunology, 9, 2640. https://doi.org/10.3389/fimmu.2018.02640
This dynamic between bacteria and the immune system is well-illustrated in this figure, highlighting some bacteria and which cells they may influence:
Budden, K., Gellatly, S., Wood, D. et al. Emerging pathogenic links between microbiota and the gut–lung axis. Nat Rev Microbiol 15, 55–63 (2017). https://doi.org/10.1038/nrmicro.2016.142
Li, N., Ma, W. T., Pang, M., Fan, Q. L., & Hua, J. L. (2019). The Commensal Microbiota and Viral Infection: A Comprehensive Review. Frontiers in immunology, 10, 1551. https://doi.org/10.3389/fimmu.2019.01551
Because of how complex the microbiome is, and due to the level of uncertainty with it, it’s not uncommon for studies to deploy a negative method. That is, many researchers may deploy antibiotics to deplete an animal of their microbes, then challenge them with a virus and see whether or not this leads to better or worse outcomes.
Dumas, A, Bernard, L, Poquet, Y, Lugo-Villarino, G, Neyrolles, O. The role of the lung microbiota and the gut–lung axis in respiratory infectious diseases. Cellular Microbiology. 2018; 20:e12966. https://doi.org/10.1111/cmi.12966
Bakaletz L. O. (2017). Viral-bacterial co-infections in the respiratory tract. Current opinion in microbiology, 35, 30–35. https://doi.org/10.1016/j.mib.2016.11.003
One comment in this excerpt remarks that live, attenuated vaccines don’t appear to produce the same dysbiosis as an actual infection does, which runs counter to the Mina, et al. study I cited in a previous post which rebuts that comment. It’s important to consider differences in animal models, as well as dosing, as attenuated viruses may become pathogenic under certain circumstances and may be one of the necessary factors in secondary bacterial infections.
Mina, M. J., McCullers, J. A., & Klugman, K. P. (2014). Live attenuated influenza vaccine enhances colonization of Streptococcus pneumoniae and Staphylococcus aureus in mice. mBio, 5(1), e01040-13. https://doi.org/10.1128/mBio.01040-13
Sencio, V., Barthelemy, A., Tavares, L. P., Machado, M. G., Soulard, D., Cuinat, C., Queiroz-Junior, C. M., Noordine, M. L., Salomé-Desnoulez, S., Deryuter, L., Foligné, B., Wahl, C., Frisch, B., Vieira, A. T., Paget, C., Milligan, G., Ulven, T., Wolowczuk, I., Faveeuw, C., Le Goffic, R., … Trottein, F. (2020). Gut Dysbiosis during Influenza Contributes to Pulmonary Pneumococcal Superinfection through Altered Short-Chain Fatty Acid Production. Cell reports, 30(9), 2934–2947.e6. https://doi.org/10.1016/j.celrep.2020.02.013




Excellent article! I’m going to send it to my GP. Definitely not enough thought to this axis in the body. Meanwhile, I’m still taking my liposomal glutathione pills :) Thank you for everything Modern Discontent!
When I caught omicron, I barely had any sign of illness but I had a couple weeks of gut difficulty. You brought up a lot of good possibilities as to why that is. I try to make homemade sauerkraut regularly, and I feel so much better when I eat it every day.