Japanese pharma company Shionogi undergoing clinical trials for their own COVID protease inhibitor
A preliminary examination into the new drug.
This was an article I worked on a few weeks prior but shelved at the time, but I’m releasing it to provide some news on some of the COVID therapeutics in the works and things to look out for.
There’s no doubt that the number of “formally approved” therapeutics for COVID are extremely scant, with Molnupiravir and PAXLOVID being the two prevailing drugs that have been given the seal of approval by the alphabet organizations.
Concerns over viral rebound from PAXLOVID (which can also appear with Molnupiravir as well) and concerns over selective pressure on the virus has stirred up plenty of controversy.
Prior to COVID it was unheard of for doctors to provide a monotherapy for different viral infections, and even many different groups such as the FLCCC have treated COVID with a cocktail therapy, so the fact that these drugs have been considered viable treatments on this argument alone is both concerning and, quite frankly, ridiculous.
However, several weeks ago I covered that one possible option would be a combination therapy comprised of both Molnupiravir and PAXLOVID based on findings in nonhuman primate models, which at least points to the significance of combination therapies over monotherapy options that are being pushed.
But in any case many questions have been raised based on the effectiveness of these drugs and the confounding variables that are associated with the use of these drugs.
Given all of these controversies, several pharmaceutical companies continue to push forward in producing COVID therapeutics.
One example is a Japanese pharmaceutical company named Shionogi which has been investigating their own protease inhibitor with hopes of starting global Phase III clinical trials soon.
The drug in question, named Ensitrelvir, or brand name Xocova was synthesized and investigated with the help of researchers from Hokkaido University.
The research here is preliminary but I wanted to post a bit of what I have found so far.
Ensitrelvir Background
Ensitrelvir has been under investigation since 2021, with the synthesis, discovery, and initial tests of the drug being published in a lengthy article from Unoh, et al.1 formally released in May of this year.
An overview diagram provided from this study can be seen below (S-217622 is the designation given to Ensitrelvir as an investigatory molecule):
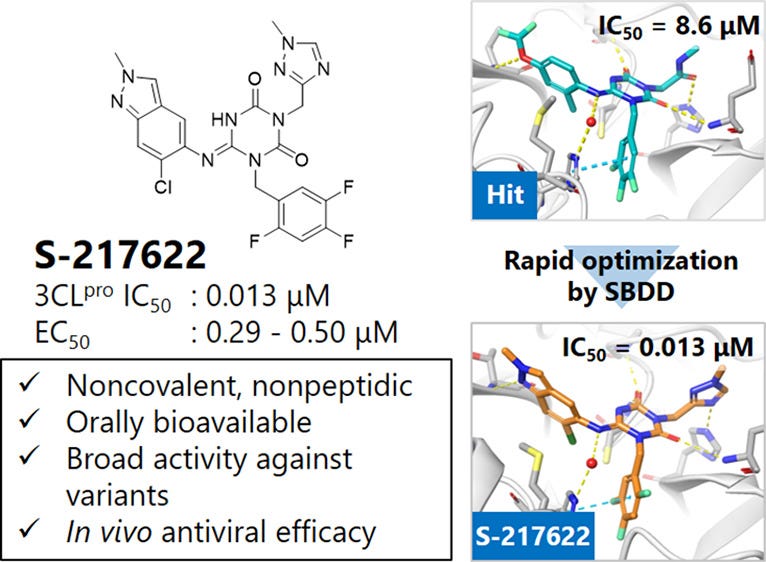
As noted in the top-left image Ensitrelvir is a rather bulky molecule comprised of several heterocyclic ring structures branching off. However, this isn’t out of the ordinary for protease inhibitors.
Protease inhibitors work by having a ton of different functional groups that branch off of the molecule and can interact with the amino acids and sugars from the enzyme. This interaction is best imagined as being akin to Velcro full of different "sticking points”. Velcro is only as good as the number of sticking points that occur, and if something blocks this sticking such as a piece of “lint” then the two pieces of Velcro cannot come together.
Protease inhibitors, and heck even antibodies work in a similar manner by serving as the “lint” that sticks to one side of the Velcro (the antigen or enzyme) that then prevents the attachment of the other piece of Velcro (the receptor or the substrate).
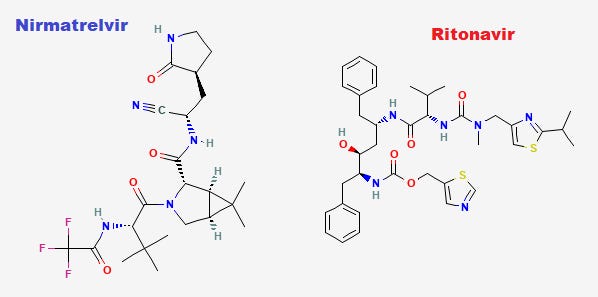
Note that Nirmatrelvir (left) and Ritonavir (right), which was originally considered a protease inhibitor, are bulky molecules as well and try to take advantage of the Velcro-like sticking to enzymes.
Researchers first created various precursor molecules and tested their binding affinity, leading to the first compound in the top-right corner of the diagram showing a decent degree of binding albeit at a relatively high dose as indicated by the IC50 concentration (the inhibitory concentration where 50% of the enzymes become inhibited).
With additional modifications the researchers eventually stumbled upon Ensitrelvir which showed a high binding affinity and inhibition of the viral protease at very low doses in vitro.
This inhibitory action is due to the combination of hydrogen bonding between the drug and the protease (shown in yellow dashed lines below) as well as a unique π-π stacking interaction between the trifluoro-phenyl ring of Ensitrelvir and the His41 residue of the protease (in cyan) which is a critical amino acid for the catalytic reaction of the enzyme.2
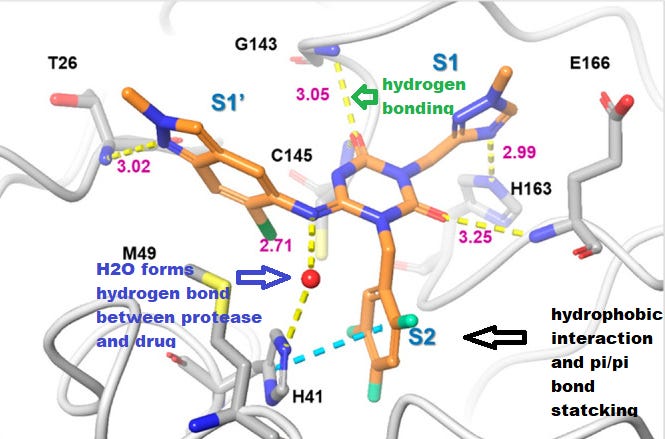
However, unlike Nirmatrelvir the interaction between Ensitrelvir and the protease are comprised of noncovalent interactions such as hydrogen bonding, hence the designation of this molecule as a noncovalent inhibitor.
In general, noncovalent interactions are generally weaker than covalent interactions. If noncovalent interactions make up the Velcro-like interaction between two molecules, covalent bonds are the link in the chain which may be either difficult or impossible to remove.
For example, it’s assumed that Nirmatrelvir’s mechanism of action includes a weak covalent interaction between the catalytic Cys145 residue of the protease and the nitrile group of Nirmatrelvir forming a thioimidate adduct (Zhao, et. al.3):
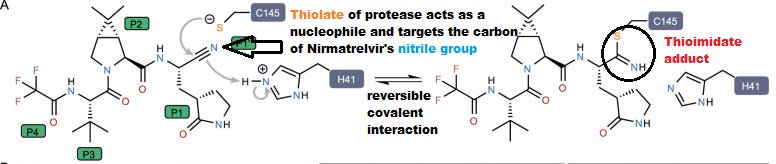
Based on these differences alone, it could be assumed that Ensitrelvir may not be as effective as Nirmatrelvir due to this weaker interaction, however a look into a comparison between the inhibitory concentration (IC50) between Ensitrelvir and Nirmatrelvir may provide additional insights (this is a preliminary assessment and I may have a greater examination in the near future).
This, therefore, raises some questions about the possible efficacy of this drug given the fact that the other drugs approved are under heavy scrutiny. This is a preliminary look and so I will look deeper in the near future.
One thing that makes Ensitrelvir unique is that, unlike Nirmatrelvir which is paired with a Cytochrome P450 Inhibitor Ritonavir, Ensitrelvir operates as its own P450 Inhibitor.
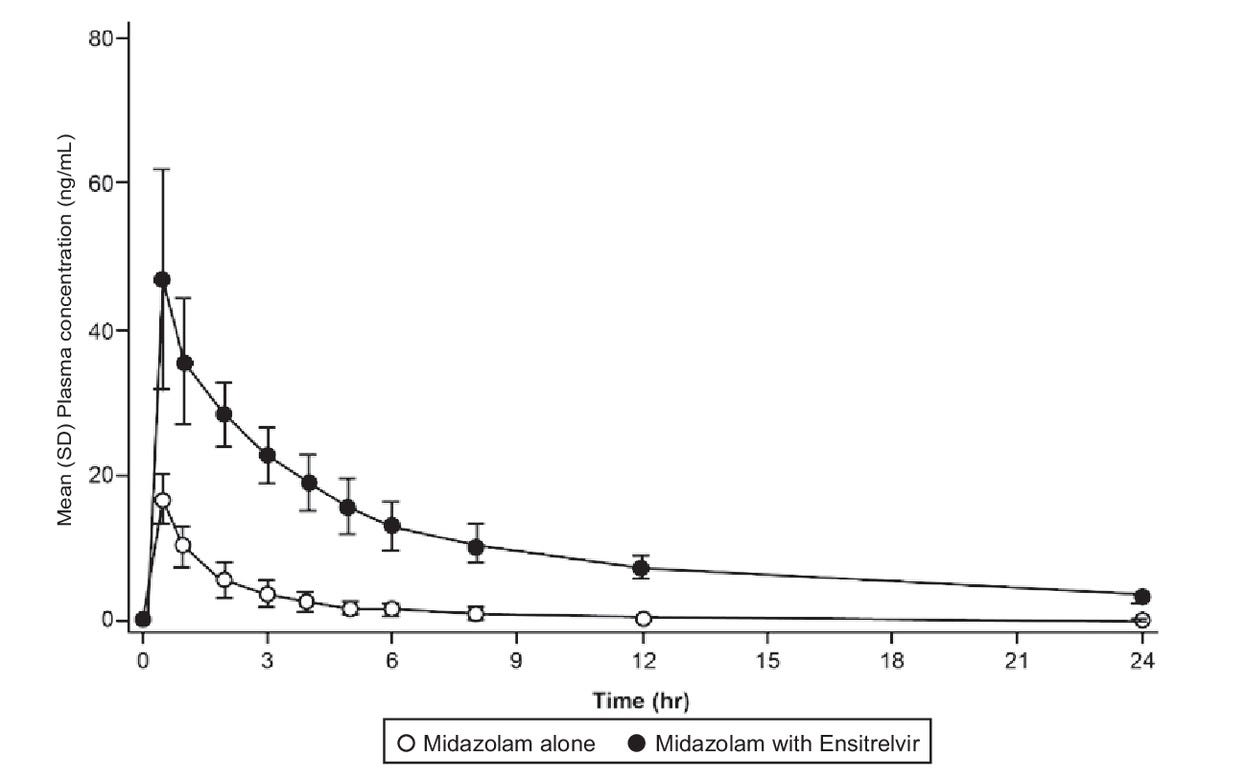
In a Phase I clinical trial4 for Ensitrelvir researchers provided participants with a 750 mg loading dose of Ensitrelvir followed by 2 mg of a CYP3A substrate called Midazolam on Day 6 of the treatment.
Given that Midazolam is metabolized by the CYP3A isozyme, this study was intended to indicate whether serum levels of Midazolam would be altered when given with Ensitrelvir.
The results showed a higher serum concentration of Midazolam in those provided Ensitrelvir, suggesting that Ensitrelvir may increase its own serum levels by inhibiting the very enzyme that is supposed to metabolize it:
The increase in AUC0-inf of midazolam upon coadministration with ensitrelvir suggested that ensitrelvir 750/250 mg acts as a strong CYP3A inhibitor.
Therefore, Ensitrelvir can inhibit both the viral protease as well as the CYP3A isozyme, leading to an extended half-life compared to other protease inhibitors.
Preliminary Press Release
I first heard about this drug after coming across this press release from the pharmaceutical company that was released a few days ago touting the results of a clinical trial.
This study was conducted in various Asian countries, but more importantly this study was conducted in a time period where most participants were vaccinated and during the time of the Omicron outbreak (Note: lack of genetic sequencing would infer Omicron, but won’t provide confirmatory evidence to conclude Omicron, emphasis mine):
This study was conducted in patients with mild/moderate symptoms of COVID-19 and assessed clinical symptom resolution with ensitrelvir (2 dose groups; high dose and low dose), orally administered once daily for five days, compared to placebo. A total of 1,821 patients were enrolled, in Japan, South Korea, and Vietnam, irrespective of risk factors for COVID-19 progression. The majority of patients were previously vaccinated. The primary endpoint in the study was the time to resolution of five key COVID-19 symptoms (stuffy or runny nose, sore throat, cough, feeling hot or feverish, and low energy or tiredness) which are characteristic of infection with the SARS-CoV-2 Omicron variant, in patients randomized within 72 hours from the onset of symptoms.
The results were considered “statistically significant”, showing a resolution of symptoms a full day before those within the placebo group:
In this population, the median time to resolution of the five COVID-19 symptoms was significantly reduced in those treated with the low dose of ensitrelvir (the dose level submitted for approval in Japan) compared to placebo: 167.9 hours versus 192.2 hours, a statistically significant difference of 24 hours (p=0.04). In addition, with respect to the key secondary endpoint of reduction in viral RNA on day 4 (following the third dose), ensitrelvir showed a significant difference versus placebo (p<0.0001) in the Least Squares mean change from baseline in viral RNA; a reduction of more than 1.4 log10 copies/mL versus placebo, similar to the results observed in previous studies1-3.
These are preliminary results, and we should know full well to avoid such assessments without the full release of data.
There’s plenty missing here as well, including the results for the high-dose cohort as well as any possible indications of viral rebound.
And that’s something that is critical to this drug. Given that it is being administered in a majority vaccinated group of participants, we should hope that this may provide some information on the rates of viral rebound (keeping mind that such a study would require surveillance weeks/months after the initial clinical trial period).
As it stands, this drug may end up being no different than PAXLOVID in effectiveness, but it may provide some additional insights into the dynamics between immunological factors, viral factors, and pharmacological factors.
This still doesn’t address the fact that monotherapies may be critical to this viral rebound phenomenon, which up until now hasn’t been properly addressed with the current regimens deemed acceptable my medical bodies.
For now, we’ll wait and see what comes of this drug. Shionogi hopes that the results of these Phase III Asia studies will push for eventual global studies of the therapeutic, so we may be a few months away from any additional information.
If you enjoyed this post and other works please consider supporting me through a paid Substack subscription or through my Ko-fi. Any bit helps, and it encourages independent creators and journalists outside the mainstream.

Unoh, Y., Uehara, S., Nakahara, K., Nobori, H., Yamatsu, Y., Yamamoto, S., Maruyama, Y., Taoda, Y., Kasamatsu, K., Suto, T., Kouki, K., Nakahashi, A., Kawashima, S., Sanaki, T., Toba, S., Uemura, K., Mizutare, T., Ando, S., Sasaki, M., Orba, Y., … Tachibana, Y. (2022). Discovery of S-217622, a Noncovalent Oral SARS-CoV-2 3CL Protease Inhibitor Clinical Candidate for Treating COVID-19. Journal of medicinal chemistry, 65(9), 6499–6512. https://doi.org/10.1021/acs.jmedchem.2c00117
Both Cys145 and His41 residues of the protease are involved with nucleophilic cleavage of the amide backbone from peptide substrates. Essentially, these two amino acids are needed to break the peptides into smaller fragments which can then be assembled and undergo post-translational protein modification into the intended proteins.
Zhao, Y., Fang, C., Zhang, Q., Zhang, R., Zhao, X., Duan, Y., Wang, H., Zhu, Y., Feng, L., Zhao, J., Shao, M., Yang, X., Zhang, L., Peng, C., Yang, K., Ma, D., Rao, Z., & Yang, H. (2022). Crystal structure of SARS-CoV-2 main protease in complex with protease inhibitor PF-07321332. Protein & cell, 13(9), 689–693. https://doi.org/10.1007/s13238-021-00883-2
Shimizu, R., Sonoyama, T., Fukuhara, T., Kuwata, A., Matsuo, Y., & Kubota, R. (2022). Safety, Tolerability, and Pharmacokinetics of the Novel Antiviral Agent Ensitrelvir Fumaric Acid, a SARS-CoV-2 3CL Protease Inhibitor, in Healthy Adults. Antimicrobial agents and chemotherapy, e0063222. Advance online publication. https://doi.org/10.1128/aac.00632-22