Molnupiravir COVID rebound just as possible as PAXLOVID's
The currently FDA approved SARS-COV2 antivirals don't appear to eliminate the virus, raising questions as to what is happening.
Note: Images have been added to the article.
Yesterday Igor posted an article on Dr. Fauci experiencing COVID rebound after taking PAXLOVID.
It’s pretty ironic because I was looking for something to write about (I’ll be late with my June post. Apologies!). I’ve been juggling a few ideas and reading a few papers, but for some reason I just decided to see what was going on with Molnupiravir since it’s been months since I last covered the drug.
Anyways, the situation was ironic because I happened to come across the following paper1:
Well, isn’t that a bit coincidental. This paper came out last week and it suggests that Molnupiravir recipients- not just PAXLOVID- may also experience COVID rebound.
This paper is really interesting since it provides more context for this somewhat grey area, as some people speculate that PAXLOVID rebound is a possible indication of immune dysfunction caused by the vaccines. I’ve been rather reticent in making such a claim without additional evidence, and with this new finding it does muddy the waters even a bit further.
PAXLOVID is comprised of two drugs: a protease inhibitor called Nirmatrelvir and a Cytochrome P450 enzyme inhibitor called Ritonavir. The pairing of a protease inhibitor and a P450 inhibitor is nothing out of the ordinary- it’s typically found as part of the antiviral cocktail against HIV, as the P450 inhibitor reduces metabolism of the protease inhibitor and quickly elevates its serum concentration. As to its use for a respiratory infection- this is rather new territory.
However, as a protease inhibitor PAXLOVID targets the virus at a site downstream from its genetic code. This creates a problem since it doesn’t necessarily lead to eventual destruction of the virus, but instead leads to a temporary halt in its activities. Viral proteases are involved with chopping up recently translated proteins at specific sites, and so inhibition of a protease doesn’t turn off the protein production or the viral replication, but really just halts the final process.
Brian Mowrey has written extensively about this halting process as a possible explanation for viral rebound.
Molnupiravir, on the other hand, is a nucleoside analogue (an analogue of the nucleoside cytidine) and operates as a mutagen. Thus, it targets the virus during its replication process and hopefully leads to a form of mutagenic catastrophe (i.e. the virus will have accrued so many mutations that it just ceases to function properly and “dies”2 out.)
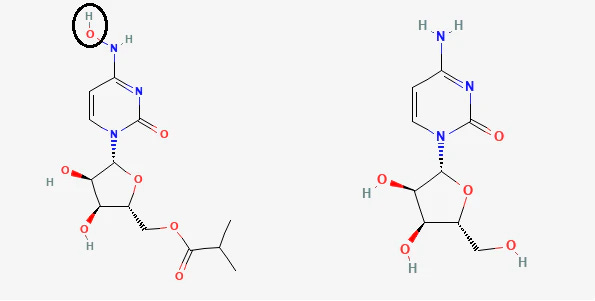
What’s important is that PAXLOVID targets a different site compared to Molnupiravir (protease vs polymerase), and so this shared rebound effect is something that shouldn’t be expected, and likely suggests something else may be happening.
With that, let’s get to the study.
COVID-19 rebound after Paxlovid and Molnupiravir during January-June 2022 (Wang, et. al. 2022)
This preprint is a retroactive study in which the researchers were interested by the current PAXLOVID rebound predicament. From there, they wanted to see if this rebound effect is occurring with Molnupiravir as well:
Recently case reports have documented that some patients treated with Paxlovid experienced rebound COVID-19 infections and symptoms 2 to 8 days after completing a 5-day course of Paxlovid3. The Centers for Disease Control and Prevention (CDC) has issued Health Alert Network Health Advisory to update the public on the potential for COVID-19 rebound after Paxlovid treatment4. However, the rate of COVID-19 rebound after Paxlovid treatment in real-world population remains unknown. In addition, questions remain as to whether COVID-19 rebound is unique to Paxlovid and whether there are patients who are more susceptible.
Considering that Molnupiravir hasn’t been as extensively used (PAXLOVID took that spotlight) it seems to have gone to the wayside and has not been prescribed to the same extent as PAXLOVID.
The researchers went through nationwide electronic health records (EHR) and looked for patients who were infected with COVID this year and were prescribed either Molnupiravir or PAXLOVID within 5 days of their infection. Patients were measured based on 3 outcomes:
a) positive COVID test;
b) COVID symptoms such as chills, fevers, cough, and shortness of breath;
c) hospitalization
What’s important to note is that this study looked at the above outcomes just 2 days after finishing the last course of either Molnupiravir or PAXLOVID, and so there’s an argument to be made as to whether this study is an examination of viral rebound or just viral persistence similar to not completing a course of antibiotics for a bacterial infection.
Since there were nearly 4X as many patients in the PAXLOVID group (11,270 vs 2,374 for Molnupiravir) patients were matched across different demographics. In these studies, matching means that you want people in one group to have a similar profile to those in another group. For example, if PAXLOVID included a white woman in her early 60s with a prior history of cancer you try to look for a patient with a similar history in the Molnupiravir group and make sure to include both in the study.
In general, most of the patients skewed older, female, and with several comorbidities, so keep that in mind when examining the data.
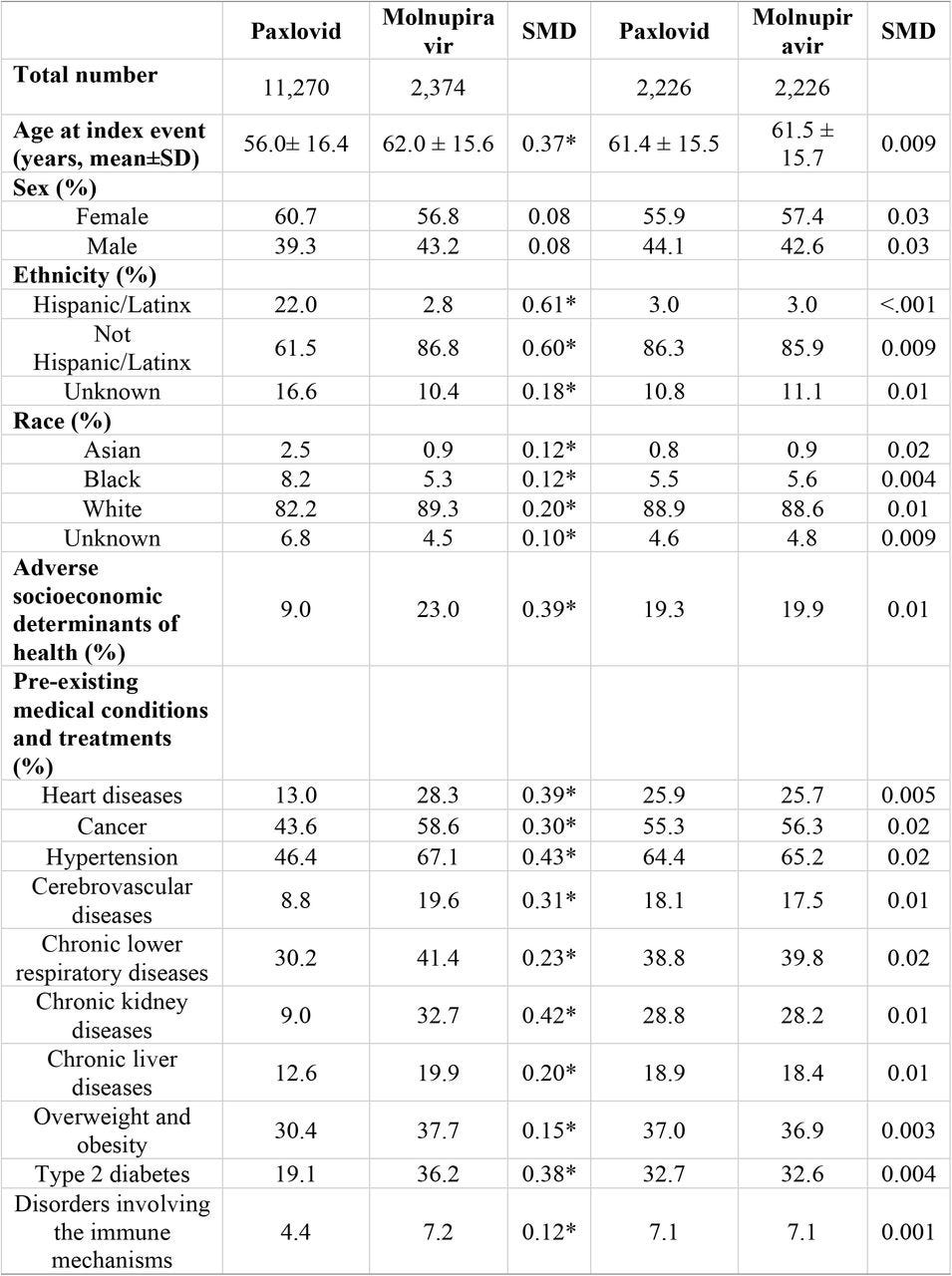
What’s really interesting about this group is that, as recorded in these patients EHRs, hardly any of these patients were vaccinated. Granted, as the researchers implied, it is likely that they were vaccinated but the information was not included in their records.

When looking at relative risk and outcomes the researchers looked at both matched and unmatched cohorts. When first looking at unmatched data Molnupiravir surprisingly showed higher rates of rebound (based on the 3 above outcomes) as compared to PAXLOVID. However, when matched the researchers argued that there was no statistical significance between the two groups, suggesting that the Molnupiravir treatment group generally skewed older with higher comorbidities (shown in the first two columns in the above patient characteristics) and likely played a factor in outcomes:
Among 11,270 patients treated with Paxlovid, 398 (3.53%) tested positive, 260 (2.31%) had COVID-19 related symptoms and 50 (0.44%) were hospitalized during the 7-day period of from 2 through 8 days after the last day of Paxlovid. COVID-19 rebound rates were higher in the 2,374 patients treated with Molnupiravir: 5.86% for rebound infections, 3.75% for rebound symptoms and 0.84% for hospitalizations (Figure 1a, top panel). As shown in Table 1, patients who took molnupiravi were older and had more comorbidities. After matching, 7-day risks for COVID-19 rebound in patients treated with Paxlovid did not differ from those treated with Molnupiravir: rebound infections (HR: 0.81, 95% CI: 0.63-1.05), rebound symptoms (HR: 0.74, 95% CI: 0.53-1.03), and hospitalizations (HR: 0.78, 95% CI: 0.40-1.51) (Figure 1b, bottom panel).
The researchers summarize this part of their study with the following:
In summary, COVID-19 rebound (infection, symptoms, and hospitalizations) occurred for both drug treatments. The risks for rebound did not differ between Paxlovid and Molnupiravir, indicating that COVID-19 rebound is not unique to Paxlovid. The rebound rates increased as the time elapsed after treatment, suggesting inadequate viral clearance by the treatments.
Lastly, the researchers wanted to examine who was experiencing rebound the most. I will not post the chart here (refer to Table 2 from the preprint) but as you can expect the rate of rebound was higher in those with higher numbers comorbidities. However, the researchers did note that rebound appears to be greater in those who were vaccinated, although remember that the actual level of those who were vaccinated was not clear and the percentages are not egregiously different based on the below table:
In summary, patients with COVID-19 rebound had higher prevalence of comorbidities that are known to be associated with higher risk for COVID-19 infection and for adverse outcomes than those without rebound. This is consistent for both Paxlovid and Molnupiravir. There were no marked demographic differences between patients with and without rebound. Patients without rebound had higher prevalence of adverse socioeconomic determinants of health. For both drugs, patients with rebound had higher vaccination rates recorded in their EHR records.

The authors note this in their discussion [additional context included]:
Our study shows that COVID-19 rebound was not unique to Paxlovid and occurred also in patients treated with Molnupiravir. The 30-day rebound rates were higher for Molnupiravir than Paxlovid: 8.59% vs 5.40% for rebound infections, 8.21% vs 5.87% for rebound symptoms and 1.39% vs 0.77% for hospitalizations. However, patients who took Molnupiravir were significantly older and had more comorbidities than those who took Paxlovid. After propensity- score matching, there were no significant differences in COVID-19 rebound risks between the two treatment cohorts. These results further suggest that rebound was not unique to Paxlovid and may be associated with persistent viral infection in some patients treated with either of these two antivirals. There has been more attention to COVID-19 rebounds following Paxlovid treatment than Molnupiravir3,4, which may be attributable to more people being treated with Paxlovid. […]
This increase [increase in rebound up to 30 days post last treatment dosage] could occur if patients had inadequate viral clearance after treatment, patients did not complete the prescribed course of treatment or developed adverse drug effects and terminated treatment, if the dose was insufficient given pharmacodynamics in that individual, if reinfection occurred, or if viruses developed resistance to the drug. Future research is required to determine if this is the case and to evaluate instances when longer treatment duration might be indicated […]
Our study showed that the vaccination rates were higher in patients who developed COVID-19 rebound than in those who did not, suggesting that vaccination was not a major contributor for COVID-19 rebound. While both drugs were tested in clinical trials that included only un-vaccinated populations, our study provided evidence that rebound occurred in largely vaccinated (89.5%) real-world populations and that rebound increased over time.
In summary, these results are rather surprising. Not only does PAXLOVID experience viral rebound but so too does Molnupiravir, which begs the question as to why this may be occurring.
Why the viral rebound?
The results of the Wang, et. al. study raises a lot more questions than it answers. With the above results, one must wonder what is going on to lead to these potentially ineffective treatments and the high rates of viral rebound.
Viral rebound with PAXLOVID
In Igor’s post he provided a screencap of a Daily Mail article. Going through that article I found out that there was a small (VERY small) study done to explain what may be happening with PAXLOVID.
After a bit of digging I was able to find the study3:
Virologic and Immunologic Characterization of COVID-19 Recrudescence after Nirmatrelvir/Ritonavir Treatment (Carlin, et. al.)
Recrudescence sounds like one of those big SAT words, but given the context you could probably figure out that it’s synonymous with recurrence or reemergence.
This study is essentially a case study in which 3 triple-vaccinated individuals travelling back to the US from South Africa tested positive for COVID and promptly given PAXLOVID. One of these individuals was found to have experienced viral rebound with COVID-like symptoms (they initially experienced improvement until after treatment was finished).
The virus was isolated from a nasopharyngeal swab and found to be of the Ba.2 Omicron variant.
Afterwards, the researchers wanted to answer if the rebound could be due to escape mutations that made PAXLOVID ineffective, or if the recipient was not fighting off the virus due to lack of neutralizing antibodies.
To answer the first question, the researchers incubated the isolated virions with Calu-3 human lung epithelial cells and incubated them with PAXLOVID. The researchers also used Remdesivir as a control reference:
Phenotypic analyses of the antiviral susceptibility of BA.2 PRSD01 (isolate), WA1/2020 (parental), B.1.617.2 (delta), BA.1 and BA.2.3 variants to NM and remdesivir were conducted in Calu-3 human lung epithelial cells. The half maximal inhibitory concentrations (IC50) of NM against BA.2 PRSD01 were 2.0, 1.8, 1.7, and 2.0 fold lower than the parental, delta, BA.1 and BA.2.3 strains, respectively (Figure 1A). As a control, we determined the IC50 for remdesivir, a drug to which this individual was not exposed, for each strain. The remdesivir IC50 for BA.2 PRSD01 was 2.0, 1.8, 1.1, and 1.3 higher than the parental, delta, BA.1 and BA.2.3 strains respectively (Figure 1A).

The above is an infection curve and measures the level of a drug needed to inhibit infection of cells. As you can imagine, higher levels of a drug should coincide with a lower rate of infection. From this we can extrapolate that PAXLOVID should still be considered an effective treatment, although Remdesivir appears to be more effective in this situation. We can also see that the variant isolated from the rebound individual (Ba.2 PRSD01) required lower doses in order to reduce infection. Overall, it suggests that, hypotheticall, PAXLOVID should still be effective as a therapeutic.
The next question asked by the researchers was if the rebound individual did not have a proper immune response (as measured by neutralizing antibody levels). Collecting sera from the individual the researchers looked at neutralization activity and measured it against the neutralizing capabilities of two controls (people who were vaccinated and boosted, with one control having had a prior infection).
The results of that neutralization assay are shown below:
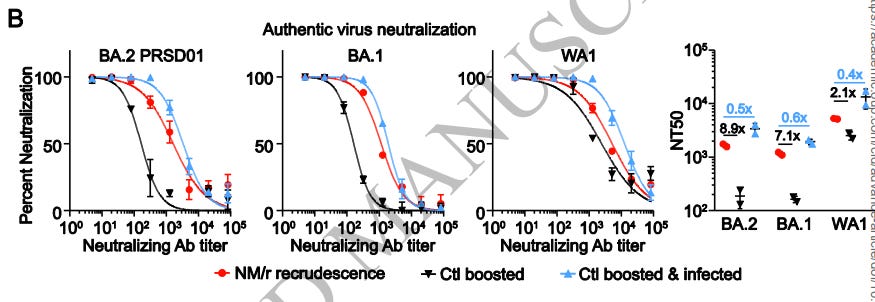
The patient’s neutralizing capabilities fell in the middle of the two controls, which suggests that neutralization antibodies were present. However, this does not explain the general scope of immunity as neither B nor T cell responses were measured. It also doesn’t include a control who was only infected and not unvaccinated, so there’s no indication of the effects of natural immunity alone. We also don’t know exactly what the proper neutralization level should be, or if the levels above coincide with actual serum concentrations.
In short, this study looked only at one individual who experienced rebound but didn’t provide much context to work with. No patient data was provided so we don’t know if comorbidities may have played a role (such as indicated in the Wang, et. al. paper).
The researchers concluded with the following (emphasis mine):
This study suggests that neither development of NM resistance nor absence of neutralizing antibody were likely causes of the observed recrudescence. The phenomenon of relapse after NM/r has been described in a limited number of individuals, all of which developed virologic rebound approximately 9-14 days after symptom onset and were likely infected with omicron variants.2 A small percentage of both NM/r and placebo-treated individuals enrolled in the primary NM/r study, EPIC-HR, which was mostly conducted before omicron emergence, also 2 experienced increasing SARS-CoV-2 RNA levels after stopping NM/r treatment at day 10 or 14 after infection.3
Our in vitro NM antiviral testing as well as sequencing, from our and other studies 2,3, have not yet identified emergence of NM resistance. Since immune responses likely contribute to eradication of replication competent virus during NM/r treatment, we evaluated neutralizing antibody responses, but found no evidence of an impaired neutralizing antibody response in this immunocompetent individual, suggesting impaired humoral immunity is not required for NM/r recrudescence. Although we were unable to measure T cell responses or serum drug concentrations, we believe the most likely possibility for the observed recrudescence is insufficient drug exposure by individual pharmacokinetics or insufficient duration. Further, the clinical significance of COVID-19 recrudescence after treatment remains unclear, so additional studies are needed to define the etiology, frequency and clinical consequences (e.g., hospitalizations, deaths, transmissions) of such recrudescence.
Overall, does this study provide much in the way of explaining this rebound? I would argue that an analysis of one individual doesn’t speak much aside from requiring further research and studies. What’s important to note here is that the researchers comment that a few of the trial participants experienced increased RNA levels after treatment completion. If anything, this would actually argue that there was evidence even from the clinical trials of viral rebound being possible.
For now, I would argue that I would need a bit more evidence to suggest that vaccinated individuals are immunocompromised and thus this plays a role in rebound. It could also be an issue of pause rather than clearance that may be an issue with PAXLOVID.
However, I would also argue that this may be another example of a rush to market that likely overlooked evidence during clinical trials that should have raised concerns, such that viral rebound was always possible but was somewhat obfuscated due to the haphazard way in which these clinical trials were conducted.
Viral rebound with Molnupiravir
Considering that not many people have been prescribed Molnupiravir (likely due to it’s highly contentious mechanism of action) we may ask whether it’s worth considering even looking into this drug.
I have written about it quite a bit in my Molnupiravir Anthology Series, so I’ll defer a bit to those prior articles for those wishing to find additional information.
Instead, I’ll focus here on the contentious MoA of Molnupiravir as a possible explanation for the rebound.
Although Molnupiravir is considered a nucleoside analogue, its modification occurs on the nitrogenous base rather than the sugar ring. Typical nucleoside analogues such as AZT and Remdesivir have modifications that halt the replication process of the virus because their modifications exist on the sugar ring (usually some modification occurs at the 3’-OH position).
In short, remember that DNA/RNA replication occurs in the 5’ → 3’ direction, and most nucleoside analogues take advantage of this biochemical mechanism by introducing modifications that halt this elongation process. In doing so, you typically end up with unusable fragments of genetic material that leads to the eventual death of the virus.
However, Molnupiravir does not halt replication. Instead, Molnupiravir requires that replication occurs. When Molnupiravir is inserted during the first viral replication process it does not do anything: it generally sits there waiting until the next replication cycle.
During the next cycle the viral polymerase uses the old genome with Molnupiravir inserted as a template to create the next RNA strand. When it comes across Molnupiravir it may read it as two possible nucleotides: if it recognizes it as a Cytidine (C) then it correctly base pairs with a G (remember that Molnupiravir is a Cytidine analogue). However, if it incorrectly recognizes Molnupiravir as a uracil (U), then the polymerase will base pair with the wrong nucleotide: Adenosine.
In doing so, Molnupiravir introduces a mutation within the next viral genome, and theoretically after several of these introductions the proteins that would be created no longer function properly and thus the virus itself ceases to function (mutagenic catastrophe).
A schematic diagram can be seen below4:

All of this may sound a bit technical, but remember that it’s important to get into the technical details to figure out what’s going on.
All this to say that Molnupiravir doesn’t necessarily lead to viral clearance. In fact, Molnupiravir works with the hope that the virus has mutated so much that it does not work properly anymore. However, that doesn’t necessarily mean that this will happen. It could be that the mutations that accrue still lead to functioning proteins, and therefore the virus can continue to propagate.
In short, Molnupiravir’s MoA is more aligned with gambling (or RNG for gamers), such that you gamble with the hope that the virus hits those right mutations to kill itself off: it doesn’t mean that this is what would actually happen.
So in some sense this makes sense why viral rebound is likely to occur with Molnupiravir because some of these mutations can still lead to competent virions that can continue to replicate.
And it’s actually this reason that there should be huge concerns with Molnupiravir.
For anyone who payed attention to the FDA Committee Meeting you would know that several of the members were concerned that Molnupiravir’s mutagenic nature may lead to more virulent, pathogenic variants.
Merck mentioned that a few of the samples they sequenced showed indications of various mutations such as the N501Y and E484K mutations commonly seen in these variants. However, it’s hard to argue whether these mutations arose naturally or through Molnupiravir- we only know of their existence because these mutations conferred greater fitness in the variants that carried these mutations.
There was also an argument to be made that viral clearance should remove any concerns since, even if concerning mutations did arise, they would eventually die off anyways.
However, this recent evidence of Molnupiravir rebound should reignite this discussion about the emergence of possibly more virulent strains through Molnupiravir treatment.
Considering that several members of the committee went so far as to argue that patients receiving Molnupiravir should isolate themselves from family members in case they create more virulent strains, shouldn’t we reexamine the utility of Molnupiravir and raise concerns that this, in fact, is now a possible reality given that Molnupiravir may not lead to viral clearance?
This creates a much more concerning scenario, and one that should warrant further investigation and analysis. PAXLOVID as a drug may only be measured on its effectiveness, but for Molnupiravir there’s a real concern that inducing mutations may confer greater fitness on the virus.
It’s fortunate that the early consternations has inhibited generally widespread use of Molnupiravir, but this should raise huge red flags that should likely take Molnupiravir off the market.
The Current Predicament
There’s quite a lot going on right now, and with all of this noise it requires a bit of contextualization.
In short, this has been a novel situation for science. No time prior to this time has such a widespread push for vaccinations and therapeutics occurred for a disease that generally does not lead to severe illness for a majority of the population.
Prior to COVID it was extremely rare to hear of someone taking an antiviral to treat the flu- one would normally let it run its course and try some fever relievers and cough suppressants. As such, there’s a case that the current antiviral predicament is one in which we had no prior example to refer to.
It’s very shocking that both of the only at-home therapeutics allowed for SARS-COV2 appear to exhibit viral rebound. It raises a lot of questions as to what is actually happening. Although there’s quite a few theories out there, it appears that this may be just another failure of the state to properly assess the effectiveness of these treatments much in the same way that the safety and efficacy of the vaccines have not been properly tested.
The clinical trials likely overlooked the possibility of viral rebound occurring, and a rush to receive EUA approval may have obfuscated the need to look for such concerns.
This is also the first time that antivirals have been used extensively at home, which raises the question as to whether this is unique to SARS-COV2 or may be a feature for any respiratory infection- there really is a serious lack of evidence since this is the first time such a undertaking has occurred.
Overall, this raises many questions as to what may be happening. But for Molnupiravir, it certainly raises questions as to whether there is any real benefit to its use now that real concerns there is a possibility that its use may lead to more virulent strains.
More research and further investigation is required, and until more evidence is provided we are all left wanting.
If you enjoyed this post and other works please consider supporting me through a paid Substack subscription or through my Ko-fi. Any bit helps, and it encourages independent creators and journalists outside the mainstream.

COVID-19 rebound after Paxlovid and Molnupiravir during January-June 2022
Lindsey Wang, Nathan A. Berger, Pamela B. Davis, David C. Kaelber, Nora D. Volkow, Rong Xu
medRxiv 2022.06.21.22276724; doi: https://doi.org/10.1101/2022.06.21.22276724
I’m not using apostrophes on dies here because of the rebound, but because viruses are technically considered nonliving. Therefore, you can’t quite kill something that has no life.
Aaron F Carlin, Alex E Clark, Antoine Chaillon, Aaron F Garretson, William Bray, Magali Porrachia, AsherLev T Santos, Tariq M Rana, Davey M Smith, Virologic and Immunologic Characterization of COVID-19 Recrudescence after Nirmatrelvir/Ritonavir Treatment, Clinical Infectious Diseases, 2022;, ciac496, https://doi.org/10.1093/cid/ciac496
Kabinger F, Stiller C, Schmitzová J, Dienemann C, Kokic G, Hillen HS, Höbartner C, Cramer P. Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis. Nat Struct Mol Biol. 2021 Sep;28(9):740-746. doi: 10.1038/s41594-021-00651-0. Epub 2021 Aug 11. PMID: 34381216; PMCID: PMC8437801.






Thanks for the thoroughness. As usual, the gold rush mentality of pHarma results in unforseen outcomes.
A new delicious post! About to start reading it!