Is myocarditis post-COVID vaccination driven by inflammation and heart-damaging immune cells?
In contrast to prior hypotheses, a new study appears to suggest that inflammation and specific immune cells, rather than autoimmunity, may be responsible for myocarditis. Part I of Barmada, et al.
I have held off on writing about this article for some time given that I needed to do a deeper dive into various aspects of this study. Unfortunately, I haven’t gotten most of it figured out yet as of the time of this writing. However, given that the first portion of this review already became rather lengthy, I found it fitting to publish the initial portion, which takes a look at the refutation made against other vaccine-related myocarditis hypotheses. The following examines a paper from Barmada, et al, which gained some attention early May as providing a counter hypotheses for carditis post-mRNA vaccination. The intent of this post isn’t to discredit this hypothesis, but to examine it within the wider context of other hypotheses and consider whether this hypothesis makes more sense relative to the other ones that have been proposed. Essentially, the following is a bifurcation- can the results provided properly argue against prior hypotheses, and does the evidence president amount to investigation into another hypothesis as proposed by Barmada, et al. The current article will examine the former, and the latter will be saved for another time.
When it comes to vaccine adverse reactions several hypotheses have circulated, including an autoimmune-mediated response or a response related to the spike protein’s pathogenicity.
Evidence supports several of these hypotheses, and yet the varied symptoms of adverse reactions makes it difficult to pinpoint one hypothesis in particular as providing the main explanation.
It’s likely that a combination of factors, dictated by the circumstances of the individual via genetic and health factors, that are driving the variety of adverse reactions seen.
With respect to myocarditis, the prevailing hypothesis of spike-driven heart damage due to spike protein interaction with ACE-II receptors, or through uptake of LNPs carrying spike mRNA has carried some weight, albeit with a few caveats.
For instance, myocarditis appears to dominate after the second dose of the mRNA vaccines. One may assume that adaptive immune sensitization after the first dose should, hypothetically, lead to binding and neutralization of spike protein in every subsequent dose. This raises a question of whether spike itself may be a culprit of myocarditis, or if other factors are driving these adverse reactions.
And, as Joomi Kim recently wrote about, not all cell types may be capable of taking up the LNPs and expressing spike, suggesting that such a phenomenon may be biased towards certain cells. This leaves the targeting of the heart in particular, up in the air as there aren’t any clear evidence of LNP uptake by cardiomyocytes or other cellular components of the heart.
So as of now the actual circumstances driving myocarditis are still yet to be fully determined.
However, a few weeks ago a study was published in Science1 which appears to refute the hypotheses outlined above (autoimmunity, in particular), and points more towards an inflammatory response that appears to drive the activation of immune cells with the capability of causing heart damage.
Now, this hypothesis isn’t a new one, as I believe several people have made this argument before, in that inflammation and the subsequent immune response may be driving heart damage.
Nonetheless, it appears that Barmada, et al. are attempting to refute any other hypothesis in lieu of their results.
The question remains whether this hypothesis will become the prevailing one, or if we shouldn’t be so quick to jump onto one hypothesis in particular.
To answer that, we'll have to take a look at this study and see what it offers.
Study Overview
Patient Breakdown
Given the inconsistencies in reports with respect to hypersensitivity and autoimmunity post-mRNA vaccination, Barmada, et al. wanted to see if they could find evidence of these hypotheses cases of myocarditis.
In order to answer this question, Barmada, et al. took blood samples from a select number of young patients and compared some of their biomarkers to healthy vaccinees.
A few points with respect to the vaccine-injured patients:
17 patients with suspected vaccine-related myocarditis/pericarditis were included in this study
87% of the patients were male
Ages ranged between 13-21, with 16.9 being the mean, suggesting a younger cohort which is typical of carditis-reported adverse reactions.
Patients appeared to have no prior medical history worth considering. They were deemed “generally healthy” prior to vaccination, which doesn’t tell us much. Table S1 notes that some of the patients were either overweight or obese, with a few being diagnosed with ADHD, anxiety, and depression, among other diseases.
Patients included were those who experienced myocarditis/pericarditis 1-4 days after receiving a second dose of BioNTech’s vaccine (2 patients included received Moderna’s SpikeVax (P12 and P14) and 1 patient received a booster (P13), as noted in Table S2). Those who had cases reported after 7 days, or who tested positive for SARS-COV2 were excluded, taking the original number of 23 patients down to 17. The former suggests that additional data is unfortunately missing aside from acute cases of myocarditis. Given that the study focuses on acute cases of myocarditis this isn’t an inherent problem- it just tells us that there’s more going on that should be investigated.
Myocarditis/pericarditis (the phrase will be interchanged with “carditis” in this post) patients were compared to both “healthy” vaccinees as well as “young, healthy” vaccinees, so no control data for unvaccinated patients exist. Keep this in mind, as the data will be relative to a group which may have biomarkers different than unvaccinated individuals.
For the most part, patients presented with signs typical of acute myocarditis/pericarditis such as nausea, congestion, shortness of breath, etc. Biomarkers were elevated including an increase in troponin and C-reactive protein levels, which suggest inflammation and possible heart damage.
Further reading of patient outcomes can be found in the paper. What’s worth examining is the clinical timeline, which notes symptom onset post-vaccination, when a patient was included in the study, and when treatment/sample collection took place:
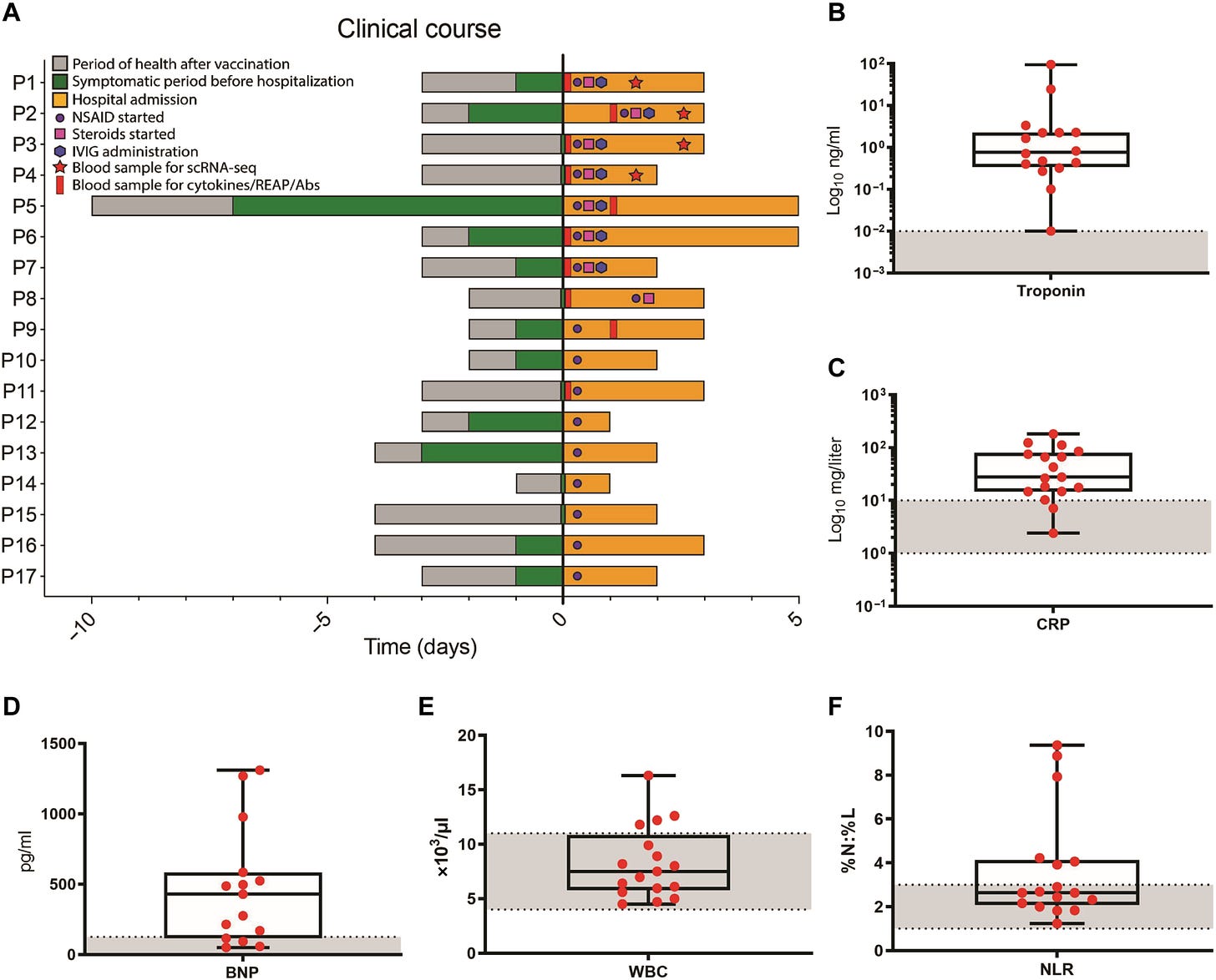
Note that some patients, such as P1-P4, P5, and P9, had some samples collected after treatment started, and thus may not reflect accurate measures of biomarkers which may be attenuated due to the use of anti-inflammatories or other treatments. For some of the analyses not all patient data was used. Note that P1-P9 were considered with respond to cytokine profiling, while characterization of immune cells via scRNA-seq (single-cell RNA-sequencing) occurred in patients P1-P4.
Refuting prior hypotheses
Antibody measures didn’t appear to infer hypersensitivity or autoimmune responses (at least as reported by the authors)
In testing prior hypotheses Barmada, et al. checked antibody levels in order to determine whether a hypersensitive or autoimmune response occurred in these myocarditis/pericarditis patients.
To quickly define these terms:
Hypersensitivity myocarditis refers to myocarditis associated with a drug or treatment, and may result in eosinophilia (over-expression of eosinophils), giant cell accumulation, or other exaggerated immune responses. In the case of Barmada, et al, hypersensitivity appears to be measured based on antibody levels, with the assumption that elevated antibody levels relative to healthy vaccinees may suggest a hypersensitive response in relation to the carditis.
Autoantibody/Autoimmunity refers to production of antibodies that target cellular structures of the heart. This has been seen in severe cases of SARS-COV2, suggesting that this may be a possibility in some vaccinees with respect to vaccine-related carditis. To examine autoimmunity, Barmada, et al. deployed something called REAP (rapid extracellular antigen profiling), which screens patient antibodies and compare it to known autoantibody profiles. REAP then provides a score based on antibody/antigen association, with higher REAP scores suggesting the detection of those autoantibodies in particular. A REAP score of at least 2 suggest a positive autoantibody reactivity response, and would suggest that the patient in question has antibodies that target a specific antigen found in the heart.
Given these hypotheses, Barmada, et al. measured antibody levels from myocarditis/pericarditis patients and compared them to healthy vaccinees.
Interestingly, antibody levels were not elevated in injured patients, and instead were lower.
Figure S2 separates antibodies based upon where they bind, which notes that vaccine-injured patients appeared to have lower levels of antibodies that targeted the S1 subunit of the spike, as well as the receptor-binding domain (RBD):
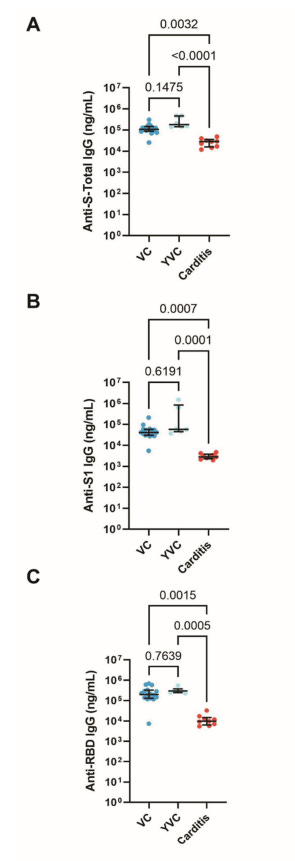
It’s curious why antibody levels appear lower in vaccine-injured individuals (Carditis). Note that healthy vaccinees (VC) had ages ranging from 26-67 while young, healthy vaccinees (YVC) had ages ranging from 26-29. In that regard, there’s an apparent mismatch in age between inured individuals and healthy vaccinees. As age and sex appear to be a critical component in myocarditis, this mismatch may not provide much in elucidating the discrepancies in antibody results.
It’s possible that age, in relation to a predisposition towards an innate immune response, may drive the lower levels of antibodies. Note that, in contrast to the carditis group, the VC group leans predominately female (13/16) while the YVC group is split evenly between male and female with a far more limited patient pool of 6.
I’ll elaborate on this problem further on, but it’s strange that the groups being used as controls aren’t representative of the group that is more inclined towards carditis, essentially confounding the results.
When comparing autoantibodies there was another strange result. REAP results appear to note a lack of autoantibody responses in carditis patients compared to YVC patients. Strangely, REAP results would suggest that several of the YVC patients have heart autoantibodies:
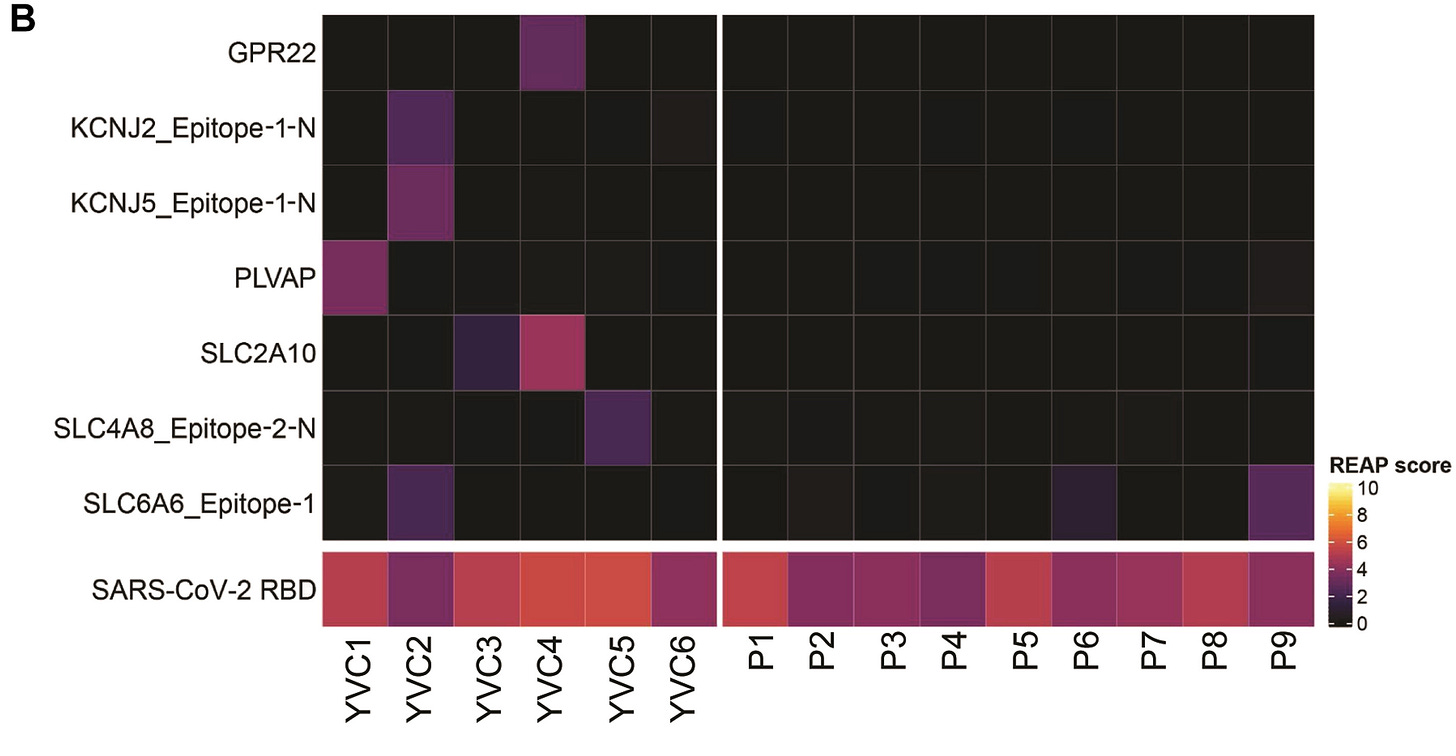
The presence of autoantibodies are based on REAP scores above 2, so it’s rather apparent that the examples shown above suggest autoantibody responses in YVC patients, which strangely isn’t elaborated on since it raises the question of whether the YVC cohort may be experiencing some form of autoimmunity-related heart damage.
So is it that autoantibody responses are occurring (at least in the YVC group), but aren’t related to carditis, or are they appearing in low enough levels that there is no pathological signs in these individuals?
When comparing the entire heatmap constructed by REAP there appears to be 2 antigens in particular that appear to score relatively high in both YVC and carditis participants (HRC and SLC16A7_Epitope-1-N2), so it’s not exactly the case that carditis patients don’t exhibit any autoantibodies.
Note that autoantibodies are assumed to be cross-reactive, in that antibody responses against the spike protein may also target antigens found in the heart. Given that carditis patients had lower antibody levels overall, this raises the question of whether one would expect to see autoantibody responses in those who don’t appear to produce as many antibodies, or if they would be detectable to a high-enough degree.
Not Quite a Refutation…
I’ll save the assessment of Barmada, et al.’s hypothesis for later on. However, given what they have presented as a refutation of prior hypotheses I don’t find these arguments to be all-too compelling.
The biggest issue I take with this study is the lack of matched cohorts, in that the healthy vaccinees are far different relative to the carditis group. Keep in mind that the current evidence of carditis post-vaccination suggests a bias towards younger men. If one were to figure out why this demographic appears to be more at risk for carditis, a properly matched cohort serving as a control should have been used. But because the control groups used lean far more female, and far older than the carditis group there are two confounding variables at play with respect to age and sex.
It’s just as likely that the results detailed above may be a result of sex-based differences, or even age as younger individuals may be more biased towards an innate immune response when dealing with a foreign antigen, relative to older individuals. Note that the mean age of the carditis group hovers around 17, while the youngest patient within the YVC group is at least 26- a near decade older than the average age of the carditis patients.
I find it hard to argue that such an age discrepancy can be waved off, as the researchers seem to do on occasion within their own article.
There’s also the fact that there doesn’t appear to be any discussion with respect to the autoantibodies being produced within the YVC group. On one hand, the lack of autoantibody formation in the carditis group may rule out an autoantibody factor with respect to carditis. On the other hand, this does raise an important question of what exactly is going on within these supposedly healthy individuals. Is there an immunological response occurring with respect to these autoantibodies being formed, or are they just more coincidental and don’t come with any pathological/clinical signs of heart damage?
These results, strangely, raise a lot more questions as to what exactly is going on with these patients. I do note that the full 81-antigen REAP assessment notes some antigens which autoantibodies seem to be formed against in both the YVC and carditis group. However, I leave that portion mostly unanalyzed given that the actual quantification of lighter shades would be difficult to determine from just the naked eye alone- the footnote below only serves to note that the REAP scores stand out with respect to some antigens, but cannot be quantitated by eyesight alone.
In that regard, I don’t consider the results examined so far from Barmada, et al. to be a refutation of autoimmunity or hypersensitivity as plausible hypotheses, although it certainly seems to raise some questions.
The biggest issue is that the design and mismatch of cohorts makes it difficult to outright make any claim of a refutation given the confounders that would be introduced.
Now, this doesn’t say anything about the evidence in support of Barmada, et al.’s hypothesis of vaccine-related carditis. This assessment will be saved for another time, although I will state that the evidence provided does raise some interesting question ideas with respect to a cytokine/immune activation aspect of carditis.
The follow-up post will hopefully come within the next few days, but will likely be driven by how much background research I need to conduct. I’d also be curious of your thoughts with the information provided above and whether you consider this to be a refutation of some of the carditis hypotheses out there.
Substack is my main source of income and all support helps to support me in my daily life. If you enjoyed this post and other works please consider supporting me through a paid Substack subscription or through my Ko-fi. Any bit helps, and it encourages independent creators and journalists such as myself to provide work outside of the mainstream narrative.

Barmada, A., Klein, J., Ramaswamy, A., Brodsky, N. N., Jaycox, J. R., Sheikha, H., Jones, K. M., Habet, V., Campbell, M., Sumida, T. S., Kontorovich, A., Bogunovic, D., Oliveira, C. R., Steele, J., Hall, E. K., Pena-Hernandez, M., Monteiro, V., Lucas, C., Ring, A. M., Omer, S. B., … Lucas, C. L. (2023). Cytokinopathy with aberrant cytotoxic lymphocytes and profibrotic myeloid response in SARS-CoV-2 mRNA vaccine-associated myocarditis. Science immunology, 8(83), eadh3455. https://doi.org/10.1126/sciimmunol.adh3455
Figure S2D doesn’t show autoantibodies in just healthy vaccinees alone, but rather there appear to be some points where both groups appear to show some evidence of autoantibody formation. However, keep in mind that the appearance of colored boxes is a qualitative assessment. The intention of pointing these specific antigens out is to note that it’s not apparent that no autoantibodies are being formed in carditis patients. However, the actual quantitation of these REAP scores cannot be done by eye alone, and thus no actual determination can be made from just looking at the below REAP scores.






My take:
(1) likely "any one of the above" across different individuals.
(2) Any way you look at it, THE JABS SHOULD BE BANNED WORLDWIDE.
There is too much corralled science out there. Where scientists seem to select an hypothesis then go all out to prove it at all cost to be the overriding one.
They look for proof not truth.
Justification for their funding.
When they start to design their studies in awareness of the intertwining nature of nature? That’s when they will be taken seriously.