What data was used for the XBB.1.5 vaccine approval?
Nothing that can show accurate safety and efficacy data, much like prior reports.
Recently the CDC approved ANOTHER round of COVID vaccines from Pfizer/BioNTech and Moderna, this time utilizing mRNA for a monovalent spike from the now-mostly extinct XBB.1.5. or ‘Kraken’ variant that appeared in early Winter 2023.

We’re way past the mythological creature naming part of the pandemic, and have moved onto astronomical (literally) naming criteria such as ‘Eris’ (EG.5) and ‘Pirola’ (BA.2.86). Or maybe they are named after mythological gods and deities. Not sure, and quite frankly it’s not the major issue with these variants.
Supposedly, out of the 14 people on the panel who voted for this approval the voting resulted in a 13-1 ‘yes’ vote for those 6 months and older. I’m rather curious who the dissenting vote was…
Strangely, Novavax, the only US-based company to produce a protein-based vaccine, has been updating their vaccine along with the mRNA manufacturers without much remarks about their updated vaccines, and even their initial Wuhan spike-based vaccine has not seen full approval. Novavax reported some of their XBB.1.5 spike results to the CDC, and yet it doesn’t appear that Novavax is on the “updated” vaccine list this coming season.
Slideshows from the different vaccine manufacturers presented to the panel can be found in the links below:
Pfizer/BioNTech: Monovalent XBB.1.5 BNT162b2 COVID-19 Vaccine
It’s sort of the same old process that we have seen before. Both Pfizer/BioNTech and Moderna utilized pseudovirus assays in order to test the neutralizing capacity of antibodies sourced from a small pool of mice given these vaccines.
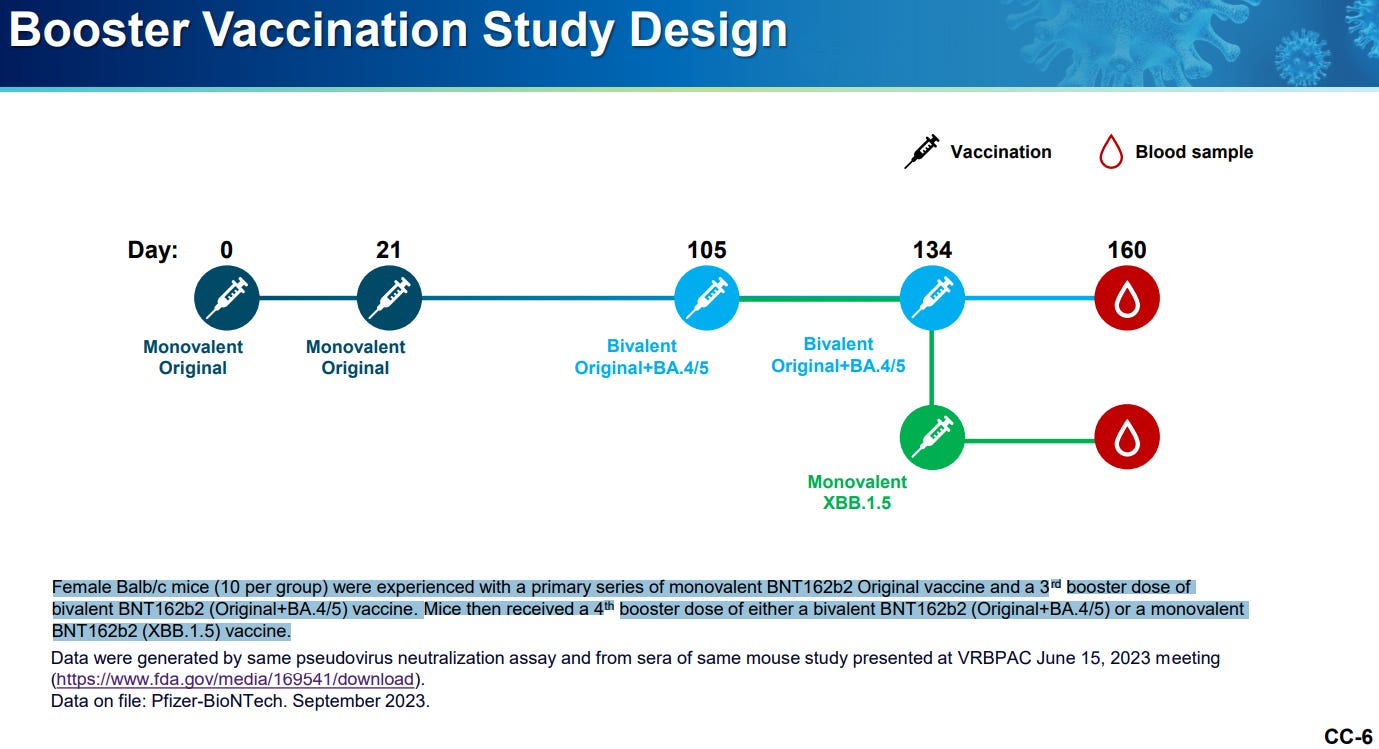
Pfizer/BioNTech seems to have gone the mouse-only route. They mention a clinical study design in their presentation, but it doesn’t seem like any results were provided. I’d have to look at the committee meeting in order to see whether any clinical trials were actually conducted for XBB.1.51.
The ‘Summary’ slide for Pfizer/BioNTech (Slide 13) seems to suggest that no clinical data was used to argue for the approval:
Note that preclinical refers to in vitro and animal models, and so this seems to argue that both no clinical studies were conducted/reported, and that vaccine manufacturers don’t seem to need to conduct clinical studies in order to update their vaccines.
This report only focused on a mere 10 mice in the XBB.1.5. group for this study (10 were given BA.4/BA.5 bivalent while 10 were given XBB.1.5):
In contrast, Moderna seems to have conducted clinical trials, albeit small, testing a monovalent XBB.1.5 spike mRNA vaccine against a BA.4/BA.5 and XBB.1.5. bivalent mRNA vaccine. Both groups had around 50 participants each, and so any serious adverse event that would be worth monitoring for would likely not appear in such a small sample size. Moderna provides some of their safety data (Slides 6 and 7), but again this is rather meaningless given the small sample size and lack of any clarity of serious adverse events.
Unfortunately, even with a clinical study only neutralizing results were provided:
This focus on antibodies has been extremely harmful, since it overlooks other aspects of the immune system. This may partially be due to the fact that dissemination of information to the public is made easy by focusing on antibodies alone, but gross simplification of the immune system isn’t a good way to inform the public. Things shouldn’t be made easier so that they appear more palatable to people.
Or maybe the CDC doesn’t require actual cellular immunity studies, and so this is all that becomes important.
Novavax provides some evidence of cellular immunity in their presentation, although this seems to be in non-clinical, rhesus macaque animal models that were only provided two doses of XBB.1.5 spike-containing vaccines rather than the “currently “recommended” vaccine schedule along with an XBB.1.5 booster. This also appears to have been done in only 5 macaques, so it doesn’t tell us much about the effects in those given more than just the XBB.1.5 vaccine.
I guess that’s better than just neutralization assays, although all of this points to the fact that robust testing hasn’t been done for even these boosters, let alone the primary vaccines and boosters.
The fact that several different vaccine manufacturers are able to seek approval using different studies should raise some concern. The argument over vaccination relies on the data being used as evidence, and if these manufacturers are looking at different data to push for their vaccine’s approval then there’s likely to be a great deal of inconsistency.
The point in posting this data isn’t to argue whether these vaccines are effective or not, because quite frankly these studies just seem too minimal to provide anything really meaningful in the real world. They certainly don’t explain how the majority of people will respond to these vaccines, and they certainly don’t provide any relevant insights into the safety and efficacy of these vaccines. This comes with the fact that so much is still not known about these adverse reactions and possible deaths following vaccination.
It’s really more of the same to be quite honest, and yet people are continuously being sold the idea that continuous vaccination will be what’s needed to deal with increasing COVID numbers, along with masking and even lockdowns in some areas.
We’re not in the same position we were 3 years ago, and we certainly should know better to fall for media trickery that may induce a sense of fear and paranoia in order to sell us a cure. The vaccines haven’t helped us out of the pandemic so far, so why would THIS be the time for it to happen?
We need more people to move on, to not feel the need to keep getting vaccinated, and to live their lives not in fear.
Substack is my main source of income and all support helps to support me in my daily life. If you enjoyed this post and other works please consider supporting me through a paid Substack subscription or through my Ko-fi. Any bit helps, and it encourages independent creators and journalists such as myself to provide work outside of the mainstream narrative.









Is SARS-CoV-2 a virus? If yes, where does it replicate?
Only in the laboratory eukaryotic cell or also in bacterial cells?
Are the bacteria in the microbiome more numerous than our cells? YES!
And does it seem normal to you that a virus passes through the microbiome layer without bacteria interacting with the virus or producing different substances than usual?
And these bacteria controls we performed and demonstrated
🔷 SARS-CoV-2 replicates first in bacteria
🔷 That orofecal transmission is most important precisely because of the bacterial involvement
🔷 That the bacteria produces toxins
🔷 That antibiotics or a combination of antibiotics can stop both replication, transmission, and toxin production and the clinical picture of patients especially in the early stages of the disease.
🔷 That the intermediate host is bacteria.
🔷 That mutations are numerous in bacteria
FDA has no leg to stand on to justify pulling any OTC supplements off of the market. Who needs testing? 😎 Let’s not forget the non-uniformity of Jab batches. What kind of GMP was that?