Remdesivir: On Facts and Fear
Even with the medical tyranny taking place we should rely on the evidence. A look at Remdesivir and some of the hysteria that has sprouted up.
Remdesivir is once again in the spotlight with recent revelations that the FDA has approved its use for SARS-COV2 in those with compromised renal function, with a Press Release from Gilead from July 14 stating the following:
This comes with ongoing controversies related to Remdesivir’s safety and efficacy, with many agencies including the WHO citing Remdesivir as being ineffective for its use against SARS-COV2 in hospitalized patients.1 This FDA-approved use for those with severe renal impairment also raises serious concerns about possible liver and kidney dysfunctions seen in those who have been administered this drug in an inpatient setting.
Given the failures of many hospital protocols to begin with, the renewed castigation of Remdesivir with this updated approval makes sense. At the same time, I am finding that arguments against Remdesivir, and really any drug, may end up relying on misinterpretations or conspiracies more than an evaluation of the data.
Now, I don’t have any love for these drugs or the people who make them, but if we were to make an argument critical about Remdesivir it should be based on sound evidence. It also requires that we hold proper standards when evaluating clinical trials.
This article won’t go into a deep dive yelling at Remdesivir, and it certainly won’t be showing any praise to the drug either. Instead, I wanted to highlight a few things that I have seen that goes more towards hysteria and conspiracy rather than critical thinking and nuanced thought.
For those who have heard this information before just a heads up that this article may seem redundant, so apologies for the possible spamming!
Again with the Ebola Trial…
For instance, if we argue that early treatment is best, it’s probably not a smart idea to provide an antiviral when someone is hospitalized and likely dealing with compromised organ function already.
It’s one of the reasons I criticized using the Ebola clinical trial when arguing against Remdesivir’s safety and efficacy. The same people arguing that there’s been a conflation of people dying from COVID and dying with COVID can’t then go around and say that 50% of the people given Remdesivir died from Remdesivir when the drug was administered in a low dosage in the trial, given over the course of more than a week, and were given so late in the disease progression that several enrolled patients died prior to receiving any treatment (hint: if you say that the risk of death increases by around 11% every day a treatment isn’t administered what do you think will happen when the average time of 1st administration is 5 days after randomization?). It’s also not as if the immunotherapies did remarkably better to begin with given that they showed a 28 mortality rate of 1/3 compared to the other treatments showing 1/2. It more than highlights that Ebola is a disease with a high case-fatality rate.
Remdesivir Retrospective: Problems with the NEJM Ebola clinical trial
Modern Discontent is a reader-supported publication. To receive new posts and support my work, consider becoming a free or paid subscriber. Given some of the current issues in analyzing studies I thou…
About Nephrotoxicity (or the importance of time)
Now, there’s the point of nephrotoxicity, which has been evaluated many times in the literature. It’s not really a contentious argument to make that there have been case reports and anecdotes from loves ones that Remdesivir use was associated with some instances of kidney failure.
The actual reason for this hasn’t been established, but what is worth considering is the fact that Remdesivir was utilized predominately for hospitalized patients and really was one of the only treatments given approval for such use. Because of that circumstance it’s likely that many patients given Remdesivir were already dealing with a host of different complications related to the infection, which may include organ dysfunction or failure.
As such, it’s quite possible that these people are in a more compromised state to begin with and may respond more negatively to certain drugs relative to nonhospitalized patients.
In some ways this is why I draw parallels between Hydroxychloroquine and Remdesivir, not due to efficacy but more due to the fact that the timing of the drug may otherwise make something that may be effective within one context (i.e. early on in the infection) into something that may be harmful. We don’t have much data on outpatient use of Remdesivir aside from a small clinical trial2, so we can’t really argue that it lacks similar complications than if used in a hospital setting given the limited data.
But even with this lack of explanation it’s important to again remember that many of these remarks on the nephrotoxicity of Remdesivir rely on its use in a hospital setting-something that wouldn’t make sense for an antiviral to begin with, and so irrespective of whether Remdesivir does or doesn’t show nephrotoxicity in an outpatient setting it doesn’t mean that it would be inherently useful within a hospital setting.
When comparing the issues with certain drugs timing is an important factor to emphasize, including whether the treatment was given in a hospital to those already in a compromised state or given in an outpatient setting with people who have an early, more mild course of the infection.
Of course, this begs the question of why Remdesivir was administered inpatient when monoclonal antibodies were provided outpatient. Part of me suspects that the early adoption of Remdesivir when everything was shut down, as well as the need for several infusions meant that there were no centers open to provide treatment. Even if they did, most patients may not be willing to make the trek and return to a center for repeat infusions required for its treatment. Even the only outpatient trial available had issues with patient compliance.
Maybe there are more reasons which others can speculate on. Further on I’ll elaborate on this weird need to administer Remdesivir via intravenous infusion, and why Remdesivir is an example of failed drug design (note: saved for the next post).
For those interested, I looked at a few of these studies examining the nephrotoxicity/cardiotoxicity of Remdesivir many months ago. Since it was from the earlier months of my Substack endeavors I didn’t scrutinize these studies to the same degree that I would now, so keep that in mind when reading.
One interesting thing is that the carrier agent, sulfobutylether-β-cyclodextrin has been implicated as a possible hepatotoxin/nephrotoxin, and the FDA’s own information on Remdesivir points the finger at this carrier agent. It’s important to note that Remdesivir came in either a lyophilized form or a solution, with the solution containing more of this carrier agent. The lyophilized form is reserved for those with kidney problems, but it’s possible that anyone may have been administered either forms of Remdesivir, meaning unknown dosing with the carrier agent.
Now, does that mean that the carrier agent is to blame? Not necessarily, and in reality I argued that both Remdesivir and the carrier agent shouldn’t be dismissed as being associated with these cases of kidney dysfunction. That article was originally paywalled, but I made it free for those interested:
Is Remdesivir Cyanogenic?
One of the reasons I chose to write this post was this strange emergence of articles suggesting that Remdesivir will metabolize to produce Hydrogen Cyanide, a well-known toxin. These groups of compounds that release hydrogen cyanide are called cyanogenic compounds, and it would be rather alarming if Remdesivir released cyanide.
Part of this assumption is derived from the active moiety of Remdesivir, which is the the addition of a cyano (also called nitrile group) to the C1’ position of the nucleoside Adenosine.3
There’s also the protecting group at the bottom called a ProTide group, but that will be discussed further on when we detail Remdesivir’s botched drug design (again, next post).
In any case, this moiety has raised some concerns, or maybe hysteria, that Remdesivir may be cyanogenic, and that those who are administered Remdesivir may be poisoned by hydrogen cyanide.
One of the issues I see with this argument is that the evidence, aside from trying to draw parallels between adverse events related to Remdesivir and hydrogen cyanide toxicity, tend to be tangential. As in, there doesn’t appear to be clear evidence provided that Remdesivir produces cyanide.
Consider that not all compounds that contain this cyano group are cyanogenic. In fact, many natural nitrile-containing compounds in nature are non-cyanogenic.4 Many drugs also contain this moiety as well5, which would then suggest that we should see evidence of hydrogen cyanide poisoning with other drugs as well.
For natural compounds that are considered cyanogenic, a certain organization of atoms seems to confer the release of cyanide, usually with the positioning of the cyano group in a position adjacent to a glycosidic bond. 6

These compounds are usually referred to as cyanogenic glycosides and are commonly found in nature. I am reminded of an episode of the Darkhorse Podcast where Bret and Heather described the need to process Cassava prior to ingesting it-something natives knew to do but the exporters/ didn’t. Cassava is an example of a plant that contains cyanogenic glycosides which must be processed in order to prevent the accumulation of cyanide during consumption.
Some cyanogenic compounds don’t need to contain this glycosidic linkage, as some nitrile-containing lipids appear to be cyanogenic.
In any case, the point of highlighting this information is to point out that a compound is not inherently going to release cyanide just because it contains a cyano moiety. It requires either a specific orientation of atoms, or it requires enzymes capable of freeing the cyanide from the molecule.
Again, if such a simple feature can induce toxicity, we should at least expect to see wider forms of toxicity with compounds, both natural and synthetic, within the literature. This is an issue where a bit of scientific knowledge may cause people to read too deep into something and infer an idea that may not be occurring, or does not have sufficient evidence.
Does this mean that Remdesivir is not completely un-cyanogenic? Not necessarily, but more that there isn’t any evidence one way or another. Pharmacokinetic data using radiolabled Remdesivir in human subjects suggests that most of Remdesivir is released either unprocessed or in the nucleoside metabolite form GS-441524 through urine.
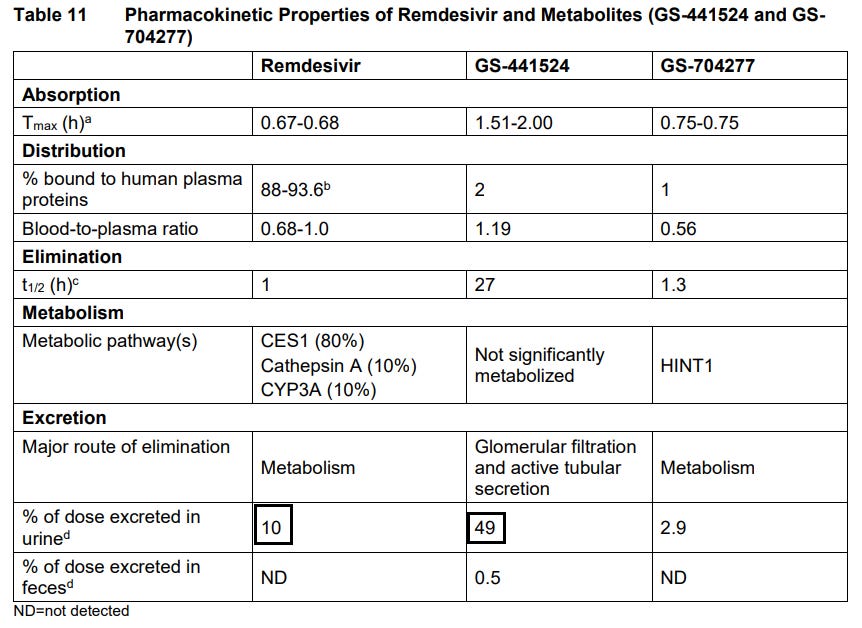
That’s still around 45ish percent unaccounted for, likely suggesting that further metabolism may have occurred to produce smaller metabolites of Remdesivir that was not tracked.
So maybe some cyanide is released, or maybe it isn’t. The point is that if one were to suggest that this is happening then hopefully such a claim comes with evidence. Unless clear evidence is provided it’s a bit of a leap to make such assumptions.
This is, unfortunately, a part of a growing problem where we may find that people may use any evidence possible to argue that COVID drugs or the vaccines are dangerous. Just because there is some truth in this statement it doesn’t mean we look for any little thing we can to substantiate the claim. Before jumping to conclusions take the time to make sense of what you are reading and discern whether there is evidence of such claims or if it’s all supposition.
Maybe this is just beating a dead horse at this point, but it’s both fascinating and concerning when there seems to be certain ideas that pop up that are validated by readers before they are substantiated. We are seeing the recirculation of spike shedding articles as well, even when that study itself has issues (the researchers never checked for actual spike, and so we can’t infer what the IgG is telling us).
Well, given how long this article has gone I will save the design of Remdesivir for the next post. Apologies for those hoping to read about that!
Substack is my main source of income and all support helps to support me in my daily life. If you enjoyed this post and other works please consider supporting me through a paid Substack subscription or through my Ko-fi. Any bit helps, and it encourages independent creators and journalists such as myself to provide work outside of the mainstream narrative.

As of 2022 the WHO has rescinded their remarks when it comes to mild or moderate COVID in those at risk of hospitalization. This seems to be based on the same evidence that the FDA has used to argue outpatient use of Remdesivir.
Gottlieb, R. L., Vaca, C. E., Paredes, R., Mera, J., Webb, B. J., Perez, G., Oguchi, G., Ryan, P., Nielsen, B. U., Brown, M., Hidalgo, A., Sachdeva, Y., Mittal, S., Osiyemi, O., Skarbinski, J., Juneja, K., Hyland, R. H., Osinusi, A., Chen, S., Camus, G., … GS-US-540-9012 (PINETREE) Investigators (2022). Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients. The New England journal of medicine, 386(4), 305–315. https://doi.org/10.1056/NEJMoa2116846
This molecule is not quite Adenosine. It lacks the C-N bond that many nucleosides have, replacing it with a pure C-C covalent bond. The purpose of this seems to be additional protective measures so that the nucleoside would not be recognized and metabolized.
Scotti C, Barlow JW. Natural Products Containing the Nitrile Functional Group and Their Biological Activities. Natural Product Communications. 2022;17(5). doi:10.1177/1934578X221099973
Fleming, F. F., Yao, L., Ravikumar, P. C., Funk, L., & Shook, B. C. (2010). Nitrile-containing pharmaceuticals: efficacious roles of the nitrile pharmacophore. Journal of medicinal chemistry, 53(22), 7902–7917. https://doi.org/10.1021/jm100762r
This article suggests that most nitrile-containing drugs do not have their nitrile groups metabolized and generally remain unchanged:
The nitrile group is quite robust and, in most cases, is not readily metabolized.4 Metabolically, the nitrile group in most nitrile-containing drugs is passed through the body unchanged.5 In cases of drug metabolism prior to elimination, the formation of glucuronides,6 conjugation with glutathione,7 N-dealkylation,8 N-acetylation,9 hydrolysis,6a-d and oxidation10 typically occurs at sites remote from the nitrile and without modification of the nitrile group.
Drugs with such moieties also appear to be evaluated as possible cyanogens, and so they may have greater scrutiny than other drugs.
Yulvianti, Meri & Zidorn, Christian. (2021). Chemical Diversity of Plant Cyanogenic Glycosides: An Overview of Reported Natural Products. Molecules. 26. 719. 10.3390/molecules26030719.





I once read a good doctor opine that clinical observation trumps RCT any day. Rem was given to anyone who stepped foot in a hospital - or so it seemed where I was. A relative that only needed hydration was admitted and hooked up for all the extra cash generating procedures.They said they couldn't just hydrate him as an outpatient. He went from high stage 3 kidney failure to 4th stage after just 2 days. They were talking dialysis and he was irate because he had no idea they snuck that in his IV. One day after stopping the Rem his numbers were back in stage 3 and the medical staff said they had never seen numbers reverse like that - and no, they would not admit it was the Rem. The other family member was not so lucky and died of kidney failure after 3 days on it. I know of many otherwise healthy people who did not fare well once the Rem was started. Take them off and they got better right away.