Pfizer's attempt at an Ozempic-like drug isn't going as planned...
Pfizer's stocks plummeted after one of their GLP-1 receptor agonists is pulled after concerning Phase 1 clinical trial findings.
Prior writings on GLP-1 Receptor Agonists for those interested:
Many pharmaceutical companies are rushing to get their own Ozempic-like drug out into the world, with several recent iterations of GLP-1 receptors agonists being formulated to be orally administered rather than the typical subcutaneous dosing regimens the likes of Ozempic and Wegovy offer.
As these drugs gain popularity, it’s no surprise to see so many companies hopping onto the bandwagon and following suit. This includes Pfizer who has attempted to develop two of their own GLP-1 receptor agonist drugs: Danuglipron and Lotiglipron, which are intended to be administered orally.
Oral administration for these classes of drugs has been challenging due to the first pass effect, in which these drugs become readily metabolized before exerting any effect on the body, so an orally available drug has been highly sought after by both patients who consider routine subcutaneous administration challenging, and by pharmaceutical manufacturers who wish to get as many people onto these drugs as possible.
However, not all is right within the state of Pfizerville. Yesterday, Pfizer announced that they had to halt clinical trials for Lotiglipron due to concerning findings during their Phase 1 clinical trial for this drug.

It doesn’t seem that many investors took too kindly to the halting of Lotiglipron, and Pfizer’s stock took around a 5% hit yesterday. Not much, but still interesting to to see happen:
So why exactly were Lotiglipron’s trials halted?
Remember that Phase 1 trials are the “safety and dosing” phase of human testing. This is where people are administered different doses of a drug to see which ones are tolerable, and also where any concerning biomarkers or physiological changes are measured that would indicate something concerning.
In the case of Lotiglipron, it appears that results from both the Phase 1 trials, as well as the ongoing Phase 2 trial, noted elevated levels of transaminases in participants:
The decision to terminate the clinical development of lotiglipron is based on pharmacokinetic data from Phase 1 drug-drug-interaction studies (C3991040 – NCT05671653 and C3991047 – NCT05788328) and laboratory measurements of elevated transaminases in these Phase 1 studies as well as the ongoing Phase 2 study C3991004 (NCT05579977). None of these participants reported liver related symptoms or side effects, there was no evidence of liver failure, and none needed treatment.
Now, what should be noted is that these clinical trials (NCT0567153 and NCT05788328) appear to be designed in order to determine drug-drug interactions when on other medications.
For instance, NCT0567153 examined whether those on birth control, the PPI Omeprazole, and the sedative Midazolam, who are then given chronic Lotiglipron will result in changes in blood measures. Both Omeprazole as well as Midazolam were administered once during the trial period. Interestingly, one arm of this trial included those given Semaglutide (Ozempic) and Midazolam concurrently.
NCT05788328 looked at the effects of 3 separate doses of the anticoagulant Dabigatran and/or the cholesterol-reducing statin Rosuvastatin on blood measures when administered with chronic Lotglipron. Note that this trial included various arms including control groups.
Given the low, limited doses for the additional drugs, as well as the lack of additional information on what was measured, it’s hard to parse exactly what drug-drug interactions may have occurred, and really is not a critical point in this post (the above information is included for those interesting in the study design).
The most important thing to highlight is the elevated transaminase levels reported.
Transaminases also known as aminotransferases1, are critical enzymes involved with the addition/removal of amine groups found on amino acids. The most common transaminases are alanine transaminase and aspartate transaminase.
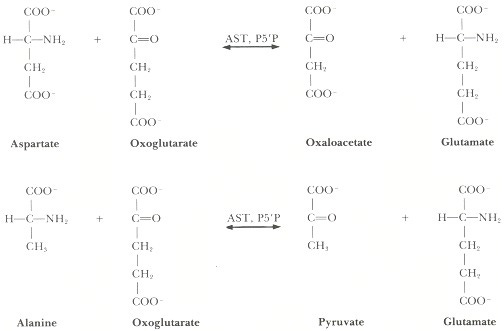
The shuffling of amines allow for necessary components for other biochemical reactions to be freed up, including those used in protein synthesis. Transaminases are ubiquitous to nearly all cells, but levels are highest within the liver.
Thus, elevated blood transaminase levels may be indicative of possible liver inflammation or damage, in much the same ways that elevated troponin levels may be a measure of possible heart inflammation/damage.
Now, note that elevated transaminase levels are not out of the ordinary for any drug. It’s common to find that many drugs come with remarks over elevated transaminase levels. However, for Pfizer to note this as a reason to halt further trials may suggest that these transaminase levels may be far higher than what is typically found. Again, a supposition on my part until more data is provided.
In Pfizer’s press release they appear to suggest that no signs/symptoms of liver damage were reported, including liver failure, and none of the participants required treatment. Upon second reading of this part, I’m a bit hesitant to make any remark that liver damage didn’t occur- it just appears that they may have gone unrecognized, or were not to such a severe degree that intervention was required, which again means that liver damage may have still occurred (a bit like myocarditis with the vaccines to be honest…)
Pfizer seems to suggest that this data may be released at another date, so we may have to be on the lookout for any other press releases or publications made in the future that can provide additional context.
Overall, what’s important to think about in lieu of this information, is again a point I have reiterated several times, in that we are in a new era of drug development where companies will be quick to get out any drug possible under the guise that this treatment will deal with some sort of public health crisis or epidemic.
We are already seeing this with Alzheimer’s immunotherapies such as Leqembi where no questions over the safety and efficacy of such drugs are allowed. As Peter Nayland Kust of All Facts Matter reminded me in one of my comments, many of these GLP-1 receptor agonists are going to be heralded as weight-loss drugs, giving regulators incentives for accelerated approval in order to deal with the growing (no pun intended) issue of obesity.
Remember, your want of a treatment may be weaponized by those who seek to rush a drug out by any means necessary.
I intended this post to include something interesting- Pfizer’s drugs lack structures similar to that of other GLP-1 receptor agonists. However, given the email limitations I’ll save that for another post.
Substack is my main source of income and all support helps to support me in my daily life. If you enjoyed this post and other works please consider supporting me through a paid Substack subscription or through my Ko-fi. Any bit helps, and it encourages independent creators and journalists such as myself to provide work outside of the mainstream narrative.

Vroon DH, Israili Z. Aminotransferases. In: Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd edition. Boston: Butterworths; 1990. Chapter 99. Available from: https://www.ncbi.nlm.nih.gov/books/NBK425/





Sounds like it may be harder than previously thought to monetize the population on hormonal birth control, brain chemistry altering antidepressants, and metabolic weight loss drugs simultaneously. My bleeding heart goes out to the shareholders. (Edit: oh and the immune altering injectable therapeutic subscription regimen, of course)
Looks like they’ll have to resort to the tried and true method of medical research and send secret teams down to the third world to experiment on peasants.