Overlooked Adverse Reactions: Hypertension Part II
Hypertension post-vaccination, and a look at the Spike Effect.
This is a continuation from the prior post, which provided a general description of hypertension, as well as the relationship between hypertension and SARS-COV2. Here, we’ll take a look at hypertension as it relates to the COVID vaccines, as well as the “Spike Effect” as a hypothesis for many of the adverse reactions seen.
If we consider that the spike protein from the vaccines are inherently similar to the spike produced by the virus we should look for COVID mimicry in those who were vaccinated.
That is to say, that if SARS-COV2 may increase the risk of hypertension we should assume that the same may be possible with those who were vaccinated.
Clinical Evidence of Hypertension Post-Vaccination
Even with limited data on adverse reactions this phenomenon has been detailed by several articles as being the “spike effect” from COVID vaccines.
Observational Data
Several data suggest that hypertension, similar to GI symptoms, are one of the most reported adverse reactions as it relates to SARS-COV2 vaccines, with some reports suggesting around 3% of vaccine recipients showing either abnormal or increased BP post-vaccination.
One of the first reported cases of hypertension post-vaccination comes from Meylan, et al.1 in which 9 vaccine recipients experienced Stage III hypertension within minutes of vaccination:
As noted by Meylan, et al. the pre-vaccination blood pressure levels were unknown. However 8 of the 9 patients apparently had well-controlled hypertension, suggestion a previous diagnoses that may have been exacerbated by vaccination.
Given the timeframe white-coat hypertension can’t be ruled out (one patient was considered to have been due to white-coat hypertension). There’s also the fact that blood pressure elevation within minutes would raise questions as to the quickness in which cells are transfected, produce spike, and are released within the circulatory system.
Although this serves as one of the first case reports with respect to BP it doesn’t tell us much about the spike effect, although other biochemical processes and the effects of other vaccine additives should also be considered.
Much of the work looking at hypertension appears to come from Angeli, et al, May 20222 and summarizes the current evidence in a Dec. 2022 review article:
Sanidas and co-workers [33] evaluated the effects of COVID-19 vaccination on BP in patients with history of controlled hypertension (defined as systolic/diastolic BP <140/90 mmHg) and healthy controls. Overall, 100 patients were enrolled [33]. All patients had BP measurements (both home and ambulatory) between the 5th and the 20th day after fully COVID-19 vaccination [33]. Patients with history of controlled hypertension showed a mean home and 24-h ambulatory BP equal to 175/97 mmHg and 177/98 mmHg, respectively [24]. Moreover, healthy controls showed a home BP of 158/96 mmHg and a 24-h ambulatory BP equal to 157/95 mmHg [33].
Ch'ng and coworkers [34] evaluated 4906 healthcare workers, recording BP when the staff members arrived at the vaccination site, immediately after vaccination, and 15–30 min later. Mean pre-vaccination systolic/diastolic BP was 130.1/80.2 mmHg and the mean changes after vaccination were +2.3/+2.4 mmHg for systolic/diastolic BP [34].
Pharmacovigilance databases were also used to evaluate this phenomenon, showing proportions of abnormal or increased BP after vaccination ranging from 1% to 3% [35], [36], [37]. Among these, a retrospective analysis involving 21,909 subjects, exhibited the largest proportion of this phenomenon [38]. Specifically, Bouhanick and co-workers investigated the BP profile of vaccinated patients and healthcare workers after the first and the second dose of COVID-19 vaccine [38]. Overall, 8121 subjects (37%) exhibited systolic and/or diastolic BP above 140 and/or 90 mmHg after the first dose. Interestingly, the majority (64%) of subjects with abnormal BP after the first injection showed a persistent abnormal BP after the second one [38].
Unfortunately, these cases also suffer from short-term measurements that also did not take baseline BP measurements prior to vaccination, and so the results do not tell us whether blood pressure was already elevated in patients prior to vaccination or may be due to vaccination (whether due to white-coat hypertension, anxiety from injection, or from the actual components of the vaccines).
The Bouhanick, et al.3 paper noted that around 60% of the participants that showed elevated blood pressure after the first dose of vaccines showed elevated levels after the second:
Finally, among the subjects with high BP after the first injection and available BP measurements at the second visit, 64 % were still hypertensive after the second one. Patients with high BP after both injections were older than those with high BP after the first one only (71 ± 14 versus 67 ± 16 years. p < 0.001). 49% were men versus 44% of those with normal BPs after the second injection (p < 0.001); their initial BP after the first injection were higher than those of the patients who were hypertensive only after the first injection (SBP/DBP: 159/83 mmHg versus 148/80 mmHg; p < 0.001). Thirty-one percent of those with grade III hypertension after the first injection are still hypertensive at this stage after the second injection. Finally, 15% of the patients who were normotensive after the first injection had high BP after the second one.
The authors refer to this as a relapse in hypertension, however given that no measures were taken between the first and second doses there could be a possibility that a few of these cases were instances of persistent hypertension.
In contrast to Bouhanick, et al. the participants in Ch’ng, et al.’s4 study consisted of much younger participants with a mean age around 33 (compared to 59 in Bouhanick, et al.) and fewer comorbidities, which have been reflected in the relatively low changes measured in this study.
Interestingly, the study from Ch’ng, et al. was conducted due to observations that healthcare workers’ blood pressures increased after vaccination, leading them to worry about the rates of hypertension post-vaccination.
Although the increase in both systolic and diastolic blood pressure appeared clinically minimal, Ch’ng, et al. did note the following:
Overall, 58 (1.02%) were admitted into the observation room either due to hypertensive urgency or complaints of giddiness. Their mean baseline systolic and diastolic blood pressures were 159.5 (SD 20.19) and 96.4 (SD 12.92), respectively. Ten (17.2%) had underlying hypertension. Eighteen (31.0%) whose complications did not improve were transferred to the Emergency Department for further monitoring and treatment as necessary.
This may suggest that those with underlying hypertension may be more at-risk of a hypertensive state. It’s important to remember that this study collected data on thousands of people and provided only the average of their blood pressure measures. It’s possible that several patients may have seen a sudden spike in their blood pressure that may have been washed out given the large sample size.
Aside from limited short-term data there doesn’t appear to be any evidence of longitudinal studies, suggested a huge blind spot in the literature.
This may be partly owed to the prior assumption that many of the adverse reactions from COVID vaccines would be transient, although such assumptions should never have been made and current evidence clearly notes that this is not the case, as many vaccinees are experiencing chronic health issues post-vaccination.
Given that hypertension may not go diagnosed until a serious event occurs that means many vaccinees may be unaware of their hypertension status. Unless seen my a clinician or taking routine measurements at home there may be a potential hypertensive crisis. Given that the US already has its own hypertensive crisis, with around 50% of US adults dealing with hypertension in some form, there’s serious concerns that many cases of hypertension are going unnoticed and undiagnosed until too late.
Case Reports
Case reports are also very limited in their mentioning of hypertension.
One case of a supposed hypertensive crisis is of a 71-year old woman who collapsed 3 days after her first mRNA vaccination. Evidence suggested an intracranial hemorrhage with a BP value of 210/110 mmHg—well above her previously measured BP values of 110/70 mmHg. Several days after the incident the women passed away (Athyros, V.G. & Doumas, M.5):
A female patient aged 71 years recently had the first dose of the Moderna anti-COVID-19 vaccine. Three days later, she collapsed without any previous symptoms. A right hemiplegia, aphasia, and agnosia were diagnosed. She had a blood pressure (BP) of 210/110 mm Hg, while her lifetime values were around 110/70 mm Hg. A computed tomography scan showed an intracranial hemorrhage in the left basal ganglia. The patient was treated with 4 intravenous (IV) doses of 0.15 mg clonidine hydrochloride and 2 IV doses of furosemide (20 mg). However, BP remained over 180/100 mm Hg, despite treatment during hospitalization. There was no thrombocytopenia or other abnormal blood tests. There was no previous medication or bleeding tendency. Nine days after the initial event she passed away, without any improvement.
Another case report notes a 60-year old woman who presented with dizziness and weakness on the right side of her body 8 hours after a dose of the ChAdOx-1 vaccine, with a reported BP value of 200/110 mmHg. In this case, the patient was known to have a prior history of hypertension. A CT scan suggests a possible brain bleed in this patient as well (Pandey, et al.6):
A-60-year female, known hypertensive with well-controlled blood pressure on treatment, presented with dizziness and weakness of the right half of body 8 hours after having received the first dose of COVID-19 vaccine (Covishield™). Examination revealed blood pressure of 200/110 mm of Hg, disorientation, right hemiplegia and right gaze paresis. CT scan head revealed left thalamic bleed with intraventricular extension. CT angiogram was normal. MRI brain and venogram did not reveal venous sinus thrombosis [Figure 1].
Complete blood count, platelet count, kidney function and liver function tests were normal. D-Dimer was 1500 ng/ml. Prothrombin test, INR and CRP levels were well within normal range. Electrocardiogram was unremarkable. The patient was given antihypertensives, decongestants and other supportive treatment. The patient did not require any neurosurgical intervention. Disorientation and gaze paresis improved over 6 days of hospital stay, she could follow verbal commands and communicate with her relatives. She was discharged with minimal residual right hemiparesis.
There may be more case reports in which an incident of hypertension was recorded, although was not considered the cause of death or part of the adverse reaction, so be aware that there may be more cases.
Also, I should point out that The Vigilant Fox wrote a piece in December 2022 highlighting VAERS reporting on hypertension. Note that the VAERS reports suggests a possible 10% rate of hypertension, as well as many reported cases of hypertension hospitalization and possible deaths.
I suggest some caution with VAERS as some of the reports may not be corroborated, although the number of reported adverse reactions should warrant further investigation to properly parse the data and figure out the actual safety signas.
This, again, raises serious issues as to why hypertension has not been look at more extensively, especially given the possibility of COVID mimicry due to the spike protein.
Of all symptoms hypertension should have been one of the prime adverse reactions to look for given the dynamics between the spike protein and ACEII.
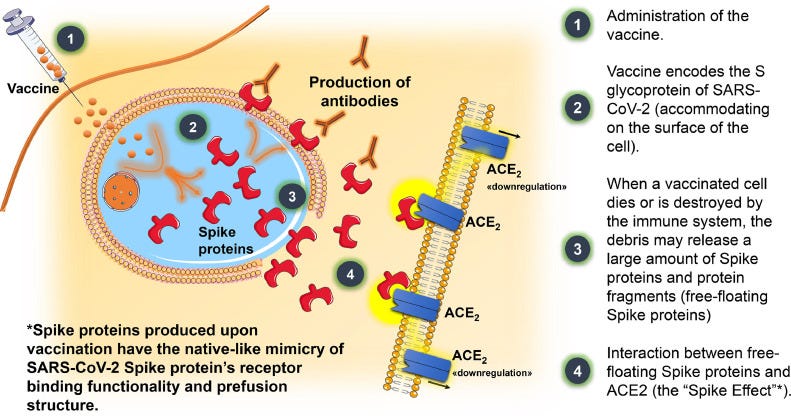
COVID-19 Vaccines and the Spike Effect
Spike and ACEII

Cheng, et al.7
Because no long-term studies appear to have been conducted, please be aware that the following is a hypothetical model that assumes continuous circulating spike due to vaccination.
Now, if we consider that the spike protein from the vaccine may be active, it should be worth considering ACEII binding and RAS dysregulation as a key component of vaccine-related hypertension.
After vaccination and transfection of host cells spike proteins and subunits may be released after cellular processing such as cell lysis. Although the clear mechanism is unknown, what is known is that circulating spike appears to be recognized given prior accounts. Circulating spike may then bind to ACEII blocking the conversion of Ang II into Ang-(1-7), as well as downregulation of ACEII. Again, Ang II may be a critical peptide hormone to examine under these circumstances.
An outline from Angeli, et al, Dec. 20228, provides a hypothetical presentation of what may be occurring after vaccination and the so-called “Spike Effect”:
It’s important to remember that circulating spike does not occur in natural infection aside from severe cases. Instead, SARS-COV2 is likely localized to the lung and the airways, only making its way into circulation after lungs become permeable through severe injury, microbiome deterioration, and deleterious inflammation.
The use of an intramuscular injection bypasses many of the natural routes of infection, and instead provides a possible direct route into circulation not seen by many infected with SARS-COV2. And so it makes sense why the spike effects seen are rather severe in nature because it circumvents typical barriers.
A review from Trougakos, et al.9 highlights this event, such that circulating spike may bind to ACEII of many cell types and may be responsible for the varied adverse reactions recorded:

Now, what’s peculiar is the incidence of adverse reactions in relation to vaccination. It appears that adverse reactions may correlate with number of vaccinations, with more adverse reactions appearing to occur after the second dose of an mRNA vaccine compared to the first dose. However, this isn’t always the case, and even in 1-dose vaccines such as the adenoviral vaccines there are severe adverse reactions noted.
Given the significance of ACEII in blood pressure and other physiological events, it’s possible that those predisposed to lower ACEII expression may suffer more severe adverse reactions as they may be unable to compensate for the downregulation of ACEII.
This may also explain a dosing relationship and the increase in adverse reactions after each additional dose. It’s possible that the first dose may lead to extensive downregulation of ACEII, in which case higher risk groups may suffer adverse reactions. The timing of the second dose may *not allow time for RAS to reach homeostasis, in which case an additional dose may perturb RAS even further.
*Changed from now to not.
Other Angiotensinases
What’s also worth noting is that ACEII is not the only enzyme responsible for converting Ang II into Ang-(1-7).
Two additional enzymes that are angiotensinases are also involved with this conversion. In particular, the enzymes prolyl oligopeptidase (POP) and prolyl carboxypeptidase (PRCP) are also involved with converting Ang II.
The role of POP and PRCP in human health is rather interesting. They appear to increase in expression among the elderly, and there’s some possible relationship between these enzymes and neurodegeneration due to their ability to cleave neuropeptides, suggesting a possible link between POP expression and age-related comorbidities10. Some evidence also suggests a relationship between these peptidases and immune function, although the evidence appears contentious11. Much is still not known about their activities, with many mechanisms elucidated via inhibition of these enzymes and examining the after effects.
As it relates to COVID vaccines, these angiotensinases appear to possibly provide a protective effect, in that they may help provide a compensatory mechanism by helping to convert Ang II and repair RAS dysfunction (Angeli, et al, Sep. 202212).

This may partially explain the difference in symptoms between the elderly and those who are young.
Given the correlation between POP/PRCP expression and age/comorbidities, it could be that younger individuals may experience the full brunt of ACEII downregulation and RAS dysfunction. This may explain the higher rates of myocarditis in young males relative to older vaccinees.
In contrast, the elderly may have elevated levels of POP/PRCP which may help convert Ang II into Ang-(1-7), providing a compensatory mechanism to help alleviate the spike effect.
Note that this hypothesis hasn’t been substantiated, only being speculated upon by Angeli and his colleagues. It would be worth investigating the breath of this effect with clinical evidence, as it may partially explain the difference in adverse reaction presentation.
It would also be interesting to see whether the vaccines may lead to elevated expression of POP,PRCP, and other angiotensinases with abilities to break down neuropeptides. This may also partially explain why neurodegenerative adverse reactions may be seen in select individuals, as upregulation of these enzymes may contribute to cognitive decline. To that point, high levels of POP appears to be found in the brain, which would substantiate (but not confirm) a possible role of POP in these adverse reactions.
Where’s long-term Hypertension Data?
In hindsight, hypertension should have been one of the most obvious adverse reactions for researchers to have looked for.
It’s quite clear that the spike protein produced by these vaccines may still bind to ACEII, and given this possibility questions should have been raised as to extent of possible hypertension in vaccinees, or whether certain groups would be at risk for more serious complications.
What’s even more striking is that there doesn’t appear to be any long-term/follow-up data within the literature, with most cases of prolonged, sustained hypertension coming from anecdotal reports from the vaccine injured or whatever can be made parsed from VAERS data.
The only data provided in the literature comes from short-term measures of blood pressure only minutes after someone receives a vaccine. It doesn’t tell us anything about the following days or weeks, and so it’s only assumed that hypertension may be transient due to no evidence providing the contrary.
This is a clear case of absence of evidence alluding to evidence of absence.
Given that hypertension can serve as a silent killer, this raises questions as to whether many of the sudden deaths seen may have proceeded hypertension. In those cases, it would mean that critical diagnoses are being overlooked which may provide insights into these deaths.
A more cynical assumption would suggest that there may be a serious hypertensive crisis occurring that is being overlooked.
As alluded to in this post it may be worth examining the level of Ang II in vaccinees as a possible marker for hypertension, as this peptide hormone may be a key component to these adverse reactions. The same may also be said for the expression of other angiontensinases such as POP which appear to play a role in neurodegeneration.
*Note the above polls are intended to just gauge the different experiences from readers. Be aware that poll results are anonymous, and so don’t be hesitant in choosing an answer that fits your experience. Also, feel free to make any remarks below as well.
If you enjoyed this post and other works please consider supporting me through a paid Substack subscription or through my Ko-fi. Any bit helps, and it encourages independent creators and journalists outside the mainstream.

Meylan, S., Livio, F., Foerster, M., Genoud, P. J., Marguet, F., Wuerzner, G., & CHUV COVID Vaccination Center (2021). Stage III Hypertension in Patients After mRNA-Based SARS-CoV-2 Vaccination. Hypertension (Dallas, Tex. : 1979), 77(6), e56–e57. https://doi.org/10.1161/HYPERTENSIONAHA.121.17316
Angeli, F., Reboldi, G., Trapasso, M., Santilli, G., Zappa, M., & Verdecchia, P. (2022). Blood Pressure Increase following COVID-19 Vaccination: A Systematic Overview and Meta-Analysis. Journal of cardiovascular development and disease, 9(5), 150. https://doi.org/10.3390/jcdd9050150
Bouhanick, B., Brusq, C., Bongard, V. et al. Blood pressure measurements after mRNA-SARS-CoV-2 tozinameran vaccination: a retrospective analysis in a university hospital in France. J Hum Hypertens 36, 580–581 (2022). https://doi.org/10.1038/s41371-021-00634-0
*Tozinameran is Comirnaty, or Pfizer/BioNTech’s mRNA vaccine.
Ch'ng CC, Ong LM, Wong KM. Changes in Blood Pressure After Pfizer/Biontech Sars-Cov-2 Vaccination. Research Square; 2022. DOI: 10.21203/rs.3.rs-1018154/v1.
Athyros VG, Doumas M. A Possible Case of Hypertensive Crisis With Intracranial Haemorrhage After an mRNA Anti-COVID-19 Vaccine. Angiology. 2022;73(1):87-87. doi:10.1177/00033197211018323
Pandey, S., Garg, R. K., Malhotra, H. S., Kumar, N., & Uniyal, R. (2022). Accelerated Hypertension and Intracerebral Haemorrhage Following First Dose of ChAdOx1-nCOV-19 Vaccination. Annals of Indian Academy of Neurology, 25(2), 322–323. https://doi.org/10.4103/aian.aian_714_21
Cheng, H., Wang, Y., & Wang, G. Q. (2020). Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. Journal of medical virology, 92(7), 726–730. https://doi.org/10.1002/jmv.25785
Angeli, F., Zappa, M., Reboldi, G., Gentile, G., Trapasso, M., Spanevello, A., & Verdecchia, P. (2022). The spike effect of acute respiratory syndrome coronavirus 2 and coronavirus disease 2019 vaccines on blood pressure. European journal of internal medicine, S0953-6205(22)00433-2. Advance online publication. https://doi.org/10.1016/j.ejim.2022.12.004
Trougakos, I. P., Terpos, E., Alexopoulos, H., Politou, M., Paraskevis, D., Scorilas, A., Kastritis, E., Andreakos, E., & Dimopoulos, M. A. (2022). Adverse effects of COVID-19 mRNA vaccines: the spike hypothesis. Trends in molecular medicine, 28(7), 542–554. https://doi.org/10.1016/j.molmed.2022.04.007
Myöhänen, T. T., García-Horsman, J. A., Tenorio-Laranga, J., & Männistö, P. T. (2009). Issues about the physiological functions of prolyl oligopeptidase based on its discordant spatial association with substrates and inconsistencies among mRNA, protein levels, and enzymatic activity. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society, 57(9), 831–848. https://doi.org/10.1369/jhc.2009.953711
Waumans, Y., Baerts, L., Kehoe, K., Lambeir, A. M., & De Meester, I. (2015). The Dipeptidyl Peptidase Family, Prolyl Oligopeptidase, and Prolyl Carboxypeptidase in the Immune System and Inflammatory Disease, Including Atherosclerosis. Frontiers in immunology, 6, 387. https://doi.org/10.3389/fimmu.2015.00387
Angeli, F., Reboldi, G., Trapasso, M., Zappa, M., Spanevello, A., & Verdecchia, P. (2022). COVID-19, vaccines and deficiency of ACE2 and other angiotensinases. Closing the loop on the "Spike effect". European journal of internal medicine, 103, 23–28. https://doi.org/10.1016/j.ejim.2022.06.015






I would gather that probably a lot of people (especially younger people) wouldn't even be aware if their blood pressure was elevated (normally or post-vaccine). It's really a shame that better data couldn't have been collected (like a pre-vaccine blood pressure) because they are literally comparing the post-vaccine BP to nothing. But at least researchers are trying to explore some of these adverse effects.
It's all really good food for thought - and it's always amazing to see how different bodies react differently to these vaccines/infections. We are all truly unique.
John Campbell of YouTube fame did a complete a yellow card report after he developed hypertension after vaccination.