Oh, Terpene Tree! Oh, Terpene Tree!
A look at pine trees and the compounds that provide for the piney, reminiscent Christmas scent.
As a continuation from Sunday's post we’ll take a look at the ever-symbolic representation of the holidays, that is in the form of Christmas trees.

So iconic are these trees to Christmas that there are even tree ornaments for our trees. We even have scents in the shape of pine tree that we hang off of the rearview mirrors in our cars, although I don’t think pine-scented is commonly used. I hear black ice is popular though.
A select few evergreens make the cut for Christmas trees; usually those belonging to the pine (Pinus), spruce (Picea), and fir (Abies) genus of trees. All three genus belong to the family Pinaceae and are conifers (cone-bearing1) trees. Ironically, the family Pinaceae are referred to as the pine family, so like with all classifications just remember it can be a bit redundant (here I’ll use pine trees to refer broadly to these conifers unless otherwise stated).
Although rather difficult to tell from afar, the differences between genus lie in the needles:

An article from Fine Gardening notes how to tell the difference based on the needles:
Look for the number of needles that come out of the same spot on a twig. If a twig bears needles in groups of two, three, or five, you can safely call it a pine. If the twig carries its needles singly, it’s a good bet you’ve got a fir or a spruce. Pull off a needle, and roll it between your fingers. If it feels flat and doesn’t roll easily, it’s a fir. If the needle has four sides and, thus, rolls easily between your fingers, it’s a spruce.
Even as someone who has never had a real, natural Christmas tree, there’s something about getting that waft of that piney scent when outdoors and passing by trees that is so iconic, and so comforting.
Like with all good scents, the smell of pine trees have been readily captures by companies in various candles and air fresheners. For those of us who aren’t partial to the overly sweet and overpowering smell of typical holiday candles, that pine scent provides for a muddled, more “manly” smell so to speak.
So let’s take a look at where some of these piney, Christmasy smells come from, and see if there may be something of benefit from them aside from reminding us of childhood.
It’s Terpene Time!
As the title of this article suggests, many of the aroma-bearing compounds found in these conifers come from terpenes, a class of volatile hydrocarbon molecules derived from isoprene units and provide plants various flavors, scents, and colors.
I’ve likely talked about terpenes before, but here’s a refresher for those interested:
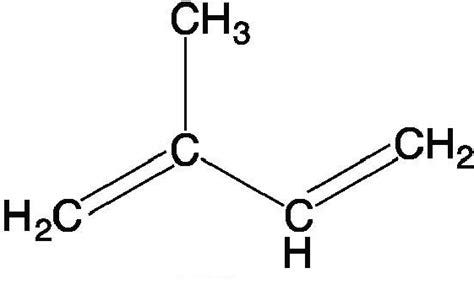
An isoprene molecule contains 5 carbons and 8 hydrogens (C5H8, as seen above). Many of the true terpenes are categorized based on the number of isoprene units that make up the molecule. Strangely, monoterpenes are made with 2 isoprene units (C10H16), sesquiterpenes with 3 isoprene units (C15H24), and so on and so forth.
Molecules that have similar structures but are slightly modified are referred to as terpene derivatives.
In particular, oxygenated forms of terpenes (bearing different oxygen functional groups) are referred to as terpenoids.
Terpenes are rather ubiquitous, and can be found in many plants and animals in multiple different forms. As noted here, they comprise many of the compounds found in essential oils derived from plants.
Also, if you hear the word terpene and think of the solvent turpentine, you are actually not off. Turpentine is derived from the distillation of resin from plants, and many of the constituent molecules are terpenes.
Now, be forewarned that if you were to look up terpenes online you’ll likely come across many articles on cannabis (likely marijuana). This is because marijuana contains many different terpenes, although so do most plants. Maybe there’s a conspiracy to get cannabis up there in the search algorithm…
Also, note that not all of these scents are derived from terpenes. Many are, and so we are being a bit broad with the association with purely terpenes. However, it’d be rather difficult to figure out every family of molecules so we are speaking broadly here when referencing terpenes as fragrant molecules.
Interestingly, the type of aromatic2 compounds found in these trees differ depending on whether the compounds are derived from the cones, pine needles, or the wood of the tree. The levels of these compounds even differ the further out form the trunk the branches go3. This makes sense, as wood from pine trees don't give off the same piney aroma as the needles.
For those with natural trees, try giving the needles and the trunk a whiff, and see if you notice an inherently different smell-just make sure no one's looking.
As to the piney smell of these trees, that is owed predominately to the molecule (+)-Bornyl acetate.
To geek out a little bit, a few years ago the America Chemical Society named (+)-Bornyl acetate as a molecule of the week during Christmas time in 2018:

(+)-Bornyl acetate is an acetate ester4 of the molecule (+)-borneol, and is likely the main constituent used in air fresheners, cleaners, perfumes and candles that help impart that fresh, piney scent.
(+)-Bornyl acetate is found in high amounts in fir and spruce trees in particular, as noted in an examination from Yang, et al.5 which looked at the constituents from silver fir essential oil (derived from twigs and leaves) and noted the following:
The oil predominantly contained bornyl acetate (30.31%), camphene (19.81%), 3-carene (13.85%), tricyclene (12.90%), dl-limonene (7.50%), α-pinene (2.87%), caryophyllene (2.18%), β-phellandrene (2.13%), borneol (1.74%), bicyclo [2.2.1]hept-2-ene,2,3-dimethyl (1.64%) and α-terpinene (1.24%).
This may be specific to the fir tree examined, so be careful in extrapolating too far into other species.
However, in an exhaustive examination of 46 different pine tree species Ionnaou, et al.6 notes that the primary aromatic compounds were α-pinene and β-pinene, but even this differed based on the different pine species.
I’m posting the abstract below, but if anyone would dare to look at all of the species and constituents, please look at the study.
The researchers note this in the abstract (emphasis mine):
The fresh needles of 46 pine species, including 37 and 17 taxa of the subgenera Pinus and Strobus, respectively, were subjected to hydrodistillation and the essential oils obtained were analyzed by means of GC–FID and GC–MS. The comprehensive analyses of the needle oils, which allowed for the identification of 161 constituents comprising the majority of the volatiles, showed significant, not only quantitative, but also qualitative differences between the samples. Monoterpenes, sesquiterpenes and diterpenes dominated the pine foliage oils, with the presence of the monoterpene hydrocarbons a- and b-pinene and the sesquiterpene hydrocarbon germacrene D characterizing most of the oils. This is the first report on the chemical composition of the essential oils of 21 pine taxa, including 15 taxa of subgenus Pinus and 6 taxa of subgenus Strobus.
So compared to fir and spruce tree needles, which may contain (+)-Bornyl acetate as the primary odorant, pine trees may be dominated by a- and b-pinene.
Unfortunately, the smell of α-pinene may not be considered too appealing, as α-pinene is considered to have a resin-like smell. In fact, α-pinene makes up most of the terpenes found in turpentine, so you can likely owe that smell to α-pinene in particular.
Aside from pine, α-pinene is also found in mint, rosemary, and of course cannabis, and may be part of what gives it that woodsy smell (in combination with other molecules that create the unique scents from different plants).
In contrast, β-pinene may impart a eucalyptus-like smell, which may be far more pleasant and soothing.
The difference between the two molecules is very small, as the only difference lies in the position of the double bond, as noted below:

Some Piney Pharmacology Overview
Many terpenes are bioactive and help provide protection to the plants that synthesize these compounds.
In particular, many of these terpenes provide antimicrobial and antioxidant defenses to plants. They may also help ward off predation from insects as well by acting as deterrents.
This has led many researchers to investigate the potential benefits of said compounds in humans, and many of the evidence suggests that these terpenes may act as antibiotics, and may provide anti-inflammatory and anti-cancer properties.
The ACS link suggests that bornyl acetate is a component of several over-the-counter cold and flu medicines, although I haven’t been able to corroborate this comment.
Interestingly, some of the evidence suggests a hypotensive, and calming antidepressant effect from various terpenes (noted in a few reviews listed below), so there could be a twofold effect when presented with Christmas trees. The imagery can invoke memories of better, more simple times. Paired with scents that may elicit antidepressant effects and we may understand why Christmas trees have such a profound effect- something holiday manufacturers use to their advantage, I bet.
On that note, a study using Korean university students7 wanted to examine the effects of smelling fir (Abies holophyla) essential oil on overall mood.
The premise of the study is rather ironic, as it was designed due to concerns over the mental health of students since they couldn’t go outdoors during lockdowns. The researchers wanted to see if exposure to essential oil from these trees would have a beneficial effect on these students and act as a sort of substitute for exposure to real trees.
The premise pretty much reads like an Air Wick commercial: if you can’t go outdoors, why not bring the outdoors inside?
Although such studies have many limitations (remember it is correlative), it appears that smelling these essential oils had a benefit on these students. However, male students appear to show sex differences in mood compared to female students, as noted in the increase in heart rate for male students in contrast to the decline for female students.
Overall, the rapid findings of terpenes as therapeutic agents has lead several of the molecules to be labeled as “molecules of interest”. However, remember to take such assertions with a grain of salt, as a lot of the evidence comes from in vitro studies and may not reflect therapeutic effects in human.
Coverage of all of these molecules will be beyond the scope of this article, but I’ll reference a few articles below for those interested. I wasn’t able to find articles that looked at bornyl acetate in a broader context, but have found several on terpenes, terpenoids, and the pinenes that may be of interest:
Bergman, et al.8: Medically Useful Plant Terpenoids: Biosynthesis, Occurrence, and Mechanism of Action
A good review for those interested in some of the biosynthetic pathways that form some of these terpenes. Some select terpenes from the monoterpenes and sesquiterpene classes are shown for their potential effects as well.
Cox-Georgian, et al.9: Therapeutic and Medicinal Uses of Terpenes.
A broad examination of terpenes as possible agents. A more easy read, although there’s a few grammatical issues with some apparently missing words, so this review may not have been edited in detail.
Allenspach, M. & Steuer, C10: α-Pinene: A never-ending story
α-Pinene has been investigated for many of its beneficial roles in cannabis. Supposedly, this molecule may work in conjunction with cannabinoids in a phenomenon called the entourage effect, in which α-Pinene may act synergistically with cannabinoids to increase their effects. However, this effect is considered controversial, and the actual quantification of α-Pinene in relation to cannabinoids is highly varied, so even if the effect is substantiated, it may actually be minimal.
Salehi, et al.11: Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature
A rather exhaustive look into these pinenes. It separates from in vitro, in vivo, and clinical studies.
Now, as to how beneficial smelling these molecules may be to our overall health is a different matter. The bioavailability of inhaled terpenes would be far different than the bioavailability of ingested terpenes, which is likely to occur frequently through the consumption of fruits and vegetables. It’s suggested that the volatile nature of some terpenes may allow them to readily access the central nervous system by crossing the blood-brain barrier. In contrast, there are some concerns as to the oxidation of terpenes in indoor settings, as the elevated ozone level indoors may cause terpenes to oxidize into more toxic substances12. However, note that both ideas are contentious at this point.
Regardless, we are readily exposed to terpenes through the foods we eat and the smells we…smell. It’s likely that many of these terpenes have effects on our bodies that we may overlook. It’s a reminder that fruits and vegetables do more than just provide us vitamins and minerals, as many of the compounds found in plants may be necessary for our overall health.
And there may be some benefit to even just smelling some of these terpenes. It may explain why we try to capitalize and capture the scents of Christmas into many products. Whether psychosomatic, or an actual consequence of biochemical pathways, the smell of pine trees may be conducive to an overall calming state.
Modernity and the current state of affairs has many people on edge. The winter months, which may lead to Seasonal Affective Disorder (SAD) due to lack of sunlight, as well as the stress of dealing with the holidays may cause us to be mentally exhausted.
So remember to take some time for yourself. Come to appreciate some of the things around you, and if possible, maybe take some time to smell the pine needles.
If you enjoyed this post and other works please consider supporting me through a paid Substack subscription or through my Ko-fi. Any bit helps, and it encourages independent creators and journalists outside the mainstream.

Conifers is derived from the Latin words conus (cone) and ferre (to bear), so conifers translate to those that bear cones.
Again, remember that aromatic here is used for the smells and fragrances, and are different than how aromatic is used in organic chemistry. Note that most terpenes actually do not fit the organic chemistry definition of “aromatic”, as many do not contain rings with delocalized electron capabilities.
Schoss, K., Benedetič, R., & Kreft, S. (2022). The Phenolic Content, Antioxidative Properties and Extractable Substances in Silver Fir (Abies alba Mill.) Branches Decrease with Distance from the Trunk. Plants (Basel, Switzerland), 11(3), 333. https://doi.org/10.3390/plants11030333
You may also see bornyl acetate referred to as a bicyclic monoterpene. The bicyclic refers to the two ring structures in the molecule, which also seen in the pinene molecules above as well.
Yang, S. A., Jeon, S. K., Lee, E. J., Im, N. K., Jhee, K. H., Lee, S. P., & Lee, I. S. (2009). Radical Scavenging Activity of the Essential Oil of Silver Fir (Abies alba). Journal of clinical biochemistry and nutrition, 44(3), 253–259. https://doi.org/10.3164/jcbn.08-240
Ioannou, E., Koutsaviti, A., Tzakou, O. et al. The genus Pinus: a comparative study on the needle essential oil composition of 46 pine species. Phytochem Rev 13, 741–768 (2014). https://doi.org/10.1007/s11101-014-9338-4
Kim, C., & Song, C. (2022). Physiological and Psychological Relaxation Effects of Fir Essential Oil on University Students. International journal of environmental research and public health, 19(9), 5063. https://doi.org/10.3390/ijerph19095063
Bergman, M. E., Davis, B., & Phillips, M. A. (2019). Medically Useful Plant Terpenoids: Biosynthesis, Occurrence, and Mechanism of Action. Molecules (Basel, Switzerland), 24(21), 3961. https://doi.org/10.3390/molecules24213961
Cox-Georgian, D., Ramadoss, N., Dona, C., & Basu, C. (2019). Therapeutic and Medicinal Uses of Terpenes. Medicinal Plants: From Farm to Pharmacy, 333–359. https://doi.org/10.1007/978-3-030-31269-5_15
Allenspach, M., & Steuer, C. (2021). α-Pinene: A never-ending story. Phytochemistry, 190, 112857. https://doi.org/10.1016/j.phytochem.2021.112857
Salehi, B., Upadhyay, S., Erdogan Orhan, I., Kumar Jugran, A., L D Jayaweera, S., A Dias, D., Sharopov, F., Taheri, Y., Martins, N., Baghalpour, N., Cho, W. C., & Sharifi-Rad, J. (2019). Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature. Biomolecules, 9(11), 738. https://doi.org/10.3390/biom9110738
Rohr A. C. (2013). The health significance of gas- and particle-phase terpene oxidation products: a review. Environment international, 60, 145–162. https://doi.org/10.1016/j.envint.2013.08.002



Fascinating read. Thank you!
Oh! For the mighty hemlock! Though I've never seen hemlock as Christmas tree commercially, I have harvested them from the forest. A droopy top is the main feature, so not good for a heavy top star, but a unique aromatic blend.