It's beginning to smell a lot like Christmas
A quick at the smells and fragrances that pervade the holiday season.

As quickly as American Thanksgiving ended did the start of the holiday season begin.
A rush for holiday sales, decorations, and family/church events comes with the onslaught of various smells and odors that we have come to relate to the season.
Even if the smells are ones we come across all throughout the year, it’s the scents that we experience during December that may resonate with us the most.
It’s no surprise, as smells can evoke fond memories and produce an overall sense of wellbeing.1 There’s an intrinsic link between smells and memories, and it’s likely why we may be so found of such smells, and also why many companies do their best to capture such scents in the form of sprays, perfumes, and candles.
Don’t be surprised if Yankee Candle entices you to buy candles by capitalizing on the memories you may have had when younger.
There’s quite a few noteworthy fragrances out there, and depending on which survey you look at the top scents can vary.
Nonetheless, the top scents are rather obvious, such as the smell of cinnamon that we douse pinecones and eggnog with, the smell of fresh-baked cookies that pervade a loved one’s house and invite copious consumption (hopefully without onlookers), or the smell that comes off of the always symbolic pine tree that takes center stage in many houses. Just make sure to water them frequently to avoid any fires!
There’s a lot to cover here, and instead of hitting every scent I will cover the select few I noted above and may expand upon at a different timepoint, so please let me know if there are other scents that I can o-pine on…
Cinnamon spice makes the holidays nice
Before the pumpkin spice mafia took over the fall season cinnamon had its own starring role in the holidays, being able to stand alone or sometimes with a little help from a friend called nutmeg.
Cinnamon has been used for centuries in cooking and as a flavoring agent, but its use as an herbal remedy may have existed just as long, as evidence suggests that cinnamon has been used for vascular diseases as well as a host of other maladies.
Some additional background, albeit a bit long, can be found below from a review by Ranasinghe, et al.2 (emphasis mine):
Cinnamon is a common spice used by different cultures around the world for several centuries. It is obtained from the inner bark of trees from the genus Cinnamomum, a tropical evergreen plant that has two main varieties; Cinnamomum zeylanicum (CZ) and Cinnamon cassia (CC) (also known as Cinnamomum aromaticum/Chinese cinnamon). In addition to its culinary uses, in native Ayurvedic medicine Cinnamon is considered a remedy for respiratory, digestive and gynaecological ailments. Almost every part of the cinnamon tree including the bark, leaves, flowers, fruits and roots, has some medicinal or culinary use. The volatile oils obtained from the bark, leaf, and root barks vary significantly in chemical composition, which suggests that they might vary in their pharmacological effects as well [1]. The different parts of the plant possess the same array of hydrocarbons in varying proportions, with primary constituents such as; cinnamaldehyde (bark), eugenol (leaf) and camphor (root) [2]. Thus cinnamon offers an array of different oils with diverse characteristics, each of which determines its’ value to the different industries. For example the root which has camphor as the main constitute, has minimal commercial value unlike the leaf and bark [3]. It is this chemical diversity that is likely to be the reason for the wide-variety of medicinal benefits observed with cinnamon.
As noted above, many of the scents associated with cinnamon spice stems from the volatile, organic compounds cinnamaldehyde and structurally similar constituents (Rao, P.V. & Gan, S.H.3):

Some evidence suggests that cinnamaldehyde can comprise nearly half of the organic compounds found in cinnamon essential oils and extracts, usually followed by another compound called eugenol:

Interestingly, cinnamaldehyde can undergo oxidation, and the conversion into cinnamic acid and other compounds may contribute to the change in color and smell of cinnamon sticks after long-term storage.
As a therapeutic agent, many of cinnamon’s compounds have been researched for their various antioxidant and anti-inflammatory properties. Most notably, the literature is rife with studies examining cinnamon as a possible aid for Type-II diabetes4.
A deeper discussion on the benefits of cinnamon will be saved for a later time. For now, it’s interesting to see another example of a well-known, commonly used spice that could hold many health benefits. All of the cinnamon containing foods may actually be beneficial health-wise, if not paired with otherwise harmful ingredients.
I also can’t speak to any possible health benefits from inhaling cinnamaldehyde that wafts off of cinnamon-scented pinecones or air fresheners, so please don’t vigorously inhale the items at in-laws houses if you want to be invited again next year…
A teaspoon of sugar helps the cookies turn brown

Aside from being a horrible play on Mary Poppin’s song, the smell of cookies can be some of the most reminiscent and fond smells.
It’s rather appropriate that today (December 4th) is apparently National Cookie Day, so what better way to celebrate the “holiday” than to bake cookies?
The smell of cookies are obviously inherent to the ingredients one puts in, and unfortunately many of the sugar cookie scents that are captured in candles and other fragrances aren’t owed specifically to capturing the essence of a baked good, but are owed to the vanilla, cinnamon, and extracts of other spices that are added to the item. It’s possible that the differences in sugar cookie smell may be due to the different ratios of oils and compounds used by different manufacturers.
Nonetheless, there are unique scents that stem from cookies, owed to the chemical reaction called a Maillard reaction.
Maillard reactions are named after Louis-Camille Maillard, who found that the gentle heating of sugars and amino acids together in water would eventually impart a yellow, brownish hue to the liquid.
Indeed, the Maillard reaction is a term for the general process of glycation, which is the reduction of amino acids and sugars that results in various compounds. In the case of Maillard reactions, the glycation occurs in the absence of enzymes and requires the use of heat. Therefore, it’s worth noting that many of the compounds derived from the Maillard reaction are not found in high abundance in nature, as heat serves as the driving force for these reactions.
This also explains why meat when reverse seared (started at low temperatures and raised to very high temperatures near the end of the cooking process) gets a very dreary, unappetizing look at lower temperatures due to a lack of the Maillard reaction taking place.
Overall, the combination of various proteins and sugars help to create various heterocyclic compounds in foods and baked goods, all contributing to the overall taste, color, and smell of cooked products.
So when someone says that baking is an exact science they aren’t quite off the mark. The proper ratio of sugar, fats, and proteins, as well as the acidity of the baked items can influence the end product by affecting the Maillard reaction process as well as the compounds formed.
This is why a steak doesn’t inherently taste or smell the same as a cookie, as noted in this Serious Eats article5:
A steak is made of muscle, which is mostly protein and water and comparatively little sugar; the high concentration of protein leads to a Maillard reaction that yields more flavor molecules and fewer aromatic ones. Cookies, on the other hand, are the opposite: With a high volume of sugar and relatively little protein, the Maillard reaction produces more aromatic compounds and fewer flavor molecules.
There’s quite a few, very IUPAC-esque named compounds6 that come about due to the Maillard reaction process that are worth noting. Most of the information here will come from a review by Adams, A., & De Kimpe, N.7 for those who are interested in a deeper assessment.
To start, one compound called 2-Acetyl-1-pyrroline, or 2-AP for short, was first discovered in cooked rice and was considered to have been one of the main contributors to the flavor and smell of grains.

However, further investigation has found that this compound can be found in various cereal and bread products as well:
Besides in cooked rice, 2-AP has also been identified among the volatiles of various other cooked cereals and cereal products: bread crust,5 toasted bread,6 corn tortillas,7 popcorn,8 cooked sweet corn products,9 extrusion cooked maize flour,10 rice cakes,11 puff pastries,12 and cooked acha (a cereal indigenous to the Sahel region in Africa).13 In all cases, 2-AP, though mostly present in low concentrations, contributes in great measure to the cereal odor notes of the food products.
2-AP contributes to the taste of popcorn, as well as the unique, toasty flavors of different food items.
Another compound formed from the Maillard reaction, with an even more convoluted name is the compound 6-Acetyl-1,2,3,4-tetrahydropyridine, or 6-ATHP:
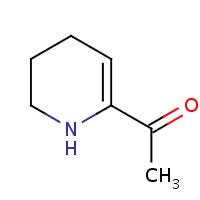
Adams, A., & De Kimpe, N. notes the following aromatic and flavor profile of 6-ATHP:
6-Acetyl-1,2,3,4-tetrahydropyridine has a typical roasty odor, resembling the flavor of crackers and popcorn, and is regarded nowadays as a very important Maillard flavor compound. Spraying week-old bread with an aqueous solution containing only 6 ppm of the sodium bisulfite complex of this compound returned a desirable fresh-bread odor to the product.64 6-Acetyl-1,2,3,4-tetrahydropyridine (6- ATHP) contributes to the aroma of several baked products: potato chips,65 bread crust,66 popcorn,8 corn tortillas,67 toast,6 and rice cakes.68 Both 2-AP and 6-ATHP contribute significantly to the flavor of bread crust, although 2-AP has the highest odor unit in wheat bread crust and 6-ATHP dominates in rye bread crust.66 Both flavor compounds are found in breadcrumbs in 30-fold lower concentrations than in the crust. This is due to the lower water activity in the outside crust, stimulating Maillard reactions.
The formation of these products are still under investigation to some degree, although the Adams, A., & De Kimpe, N. review notes that the formation of 2-AP and 6-ATPH may stem from a reduction reaction between a monosaccharide and the amino acid proline.
So when baking this holiday season note that you are engaging in chemical reactions. The proper mix of fats, sugars, water, and proteins help contribute to the overall smell and deliciousness of products.
Here, the compounds looked at aren’t specific to cookies, and it’s worth noting that many studies on the formation of Maillard reaction products tend to rely on simple sugars and amino acids, which may not encapsulate the actual protein/polysaccharide dynamic that takes place in actual cooking.
Other reactions such as caramelization can contribute to the flavors and aromas of baked goods as well, and so the combination of Maillard reaction products, the volatile compounds from various extracts and spices, and the caramelization process can coalesce into a a delectable, festive item.
The overview of pine trees shall be saved for a different time, but for now when you go on about your holiday shopping, or decide to spend some time making Christmas goodies take some time to take in some of the smells (hopefully pleasant!) around you.
Enjoy and reminisce in the memories that such smells can bring forth, and take care to spend time in making new memories.
Again, if there are any festive smells or flavors you’d like to her more of please let me know!
If you enjoyed this post and other works please consider supporting me through a paid Substack subscription or through my Ko-fi. Any bit helps, and it encourages independent creators and journalists outside the mainstream.

Bentley, P. R., Fisher, J. C., Dallimer, M., Fish, R. D., Austen, G. E., Irvine, K. N., & Davies, Z. G. (2023). Nature, smells, and human wellbeing. Ambio, 52(1), 1–14. https://doi.org/10.1007/s13280-022-01760-w
Ranasinghe, P., Pigera, S., Premakumara, G. A., Galappaththy, P., Constantine, G. R., & Katulanda, P. (2013). Medicinal properties of 'true' cinnamon (Cinnamomum zeylanicum): a systematic review. BMC complementary and alternative medicine, 13, 275. https://doi.org/10.1186/1472-6882-13-275
Rao, P. V., & Gan, S. H. (2014). Cinnamon: a multifaceted medicinal plant. Evidence-based complementary and alternative medicine : eCAM, 2014, 642942. https://doi.org/10.1155/2014/642942
Sharma, S., Mandal, A., Kant, R., Jachak, S., & Jagzape, M. (2020). Is Cinnamon Efficacious for Glycaemic Control in Type-2 Diabetes Mellitus?. JPMA. The Journal of the Pakistan Medical Association, 70(11), 2065–2069.
I haven’t corroborated which evidence was used to make this argument, and note that the use of aromatic here is not the same as how aromatic is used in organic chemistry, although the etiology for its use in organic chemistry actually derives from volatile, fragrant compounds.
IUPAC, or the International Union of Pure and Applied Chemistry, comes up with the naming practice seen in organic chemistry. Although the way that these compounds are named can seem extremely lengthy and confusing on first glance, there’s an inherent, systematic way in which these compounds are named, which is a lot more can be said for how chemists name compounds compared to biologists, who may name systems based on the order of discovery and not the order in which the biochemical reactions take place, leading to confusion amongst biology students.
Adams, A., & De Kimpe, N. (2006). Chemistry of 2-acetyl-1-pyrroline, 6-acetyl-1,2,3,4-tetrahydropyridine, 2-acetyl-2-thiazoline, and 5-acetyl-2,3-dihydro-4H-thiazine: extraordinary Maillard flavor compounds. Chemical reviews, 106(6), 2299–2319. https://doi.org/10.1021/cr040097y



Thank you! I enjoy the practical and fun organic chemistry. Natural products are so cool.