NAC, L-cysteine, Glutathione?
A review of L-cysteine derivatives with pros/cons of supplementing with each, as well as considerations for if NAC is actually necessary.
This review follows the preceding articles on NAC & COVID as well as the Pharmacology of NAC.
This review of NAC has got me going down an antioxidant rabbit hole, and I’m still just barely scratching the surface.
However, a few people have asked (and I had questions myself) if NAC supplementation would be the best, or if use of Glutathione itself would be better. Or maybe if L-cysteine would be better than NAC since it’s so widely available from foods and supplements.
We’ve essentially narrowed down the therapeutic effects of NAC to its ability to replete L-cysteine levels, which can then lead to formation of the main antioxidant GSH following a pathway below:
Because of this, wouldn’t it be easier to just take L-cysteine or GSH. And considering that L-cysteine is an amino acid can’t we make it ourselves or get some L-cysteine from food sources?
Essentially, is NAC supplementation really necessary?
There’s plenty of ways to tackle this question, and this review certainly won’t be exhaustive. Here, we’ll take a look at a few of the evidence in support of either NAC or any of the other compounds listed above.
NAC or L-cysteine?
On appearance alone, NAC is just a a fancier, acetylated (N-acetylated) version of the common amino acid L-cysteine. If L-cysteine is really the money maker here, would it make more sense to just take L-cysteine instead? Or possibly obtain L-cysteine from food sources?
The review from Pedre, et. al.1 that I've cited previously provides some explanations as to why L-cysteine may not be more suitable than NAC. Most, if not all, of the information in this section will be derived from the Pedre, et. al. review as that is one of the only robust reviews that includes the comparison between NAC and L-cysteine.
I’ll include the introductory paragraph here as Pedre, et. al. raises the same questions:
It may be asked why all the treatments and experiments were (and still are) done with NAC and not with Cys. Given the fact that NAC shares the thiol group with Cys and is a prodrug for Cys, why not directly use Cys? Arguably, Cys would look like the more obvious choice. Is this just a historical accident, i.e. somebody having started with NAC and everybody else following? Does NAC have positive properties that Cys does not have? Or does Cys have negative properties not shared by NAC?
One explanation given relates to one of the original mucolytic researchers Sheffner, who found that solutions of L-cysteine oxidized far too rapidly to form the Cys-S-S-Cys dimer called cystine (cysteine without the -e).

His observations suggested that cystine is less soluble than reduced L-cysteine and precipitated out of solution, and that this form of cystine may actually be an irritant if inhaled. That certainly would be counterproductive for its use as a mucolytic agent if it may irritate the lungs:
As Sheffner, the originator of NAC-based mucus liquefaction, explained in his initial publications, Cys was not considered ideal for therapeutic use, because in solution it oxidized rather rapidly, the oxidized form (cystine, Cys-S-S-Cys) being mostly insoluble and precipitating from the solution (Sheffner, 1963a). The crystalline cystine precipitates were feared to cause irritation in the respiratory tract.
Sheffner’s observations apparently found that NAC was more stable and had a more “agreeable” taste and odor. Not quite sure what counts as “agreeable”, but ease of administration and tolerability are huge factors to consider when designing a drug. Compliance is a huge issue in the medical field- we likely know of people who have trouble taking their medications already, including antibiotics, so anything that makes it easier for the patient is greatly valued in drug design and administration.
Pedre, et. al. further explains that multiple studies have validated the lower rate of oxidation of NAC compared to L-cysteine2:
Sheffner’s argument about the greater stability of NAC in solution is borne out by numerous experiments. Freshly prepared NAC solutions are more resistant to air oxidation than corresponding Cys solutions. In one experiment, NAC was 50% oxidized (to NAC-S-S-NAC) after 13.7 h, while Cys was already 50% oxidized (to Cys-S-S-Cys) after 5.2 h (Held & Biaglow, 1994).
It’s worth noting that this study appears to have been conducted in an in vitro setting, and therefore is not representative of the actual cellular environment. As a counterargument, it’s suggested that free L-cysteine tends to exist in the form of cystine within plasma, which raises questions as to whether other factors are needed to aid in solubilizing cystine that may not be controlled for in certain lab conditions.
Going along with this thought, one study was conducted by Whillier, et. al.3 which suggests that NAC may actually serve as a reducing agent to reduce the disulfide bonds in cystine to release reduced L-cysteine.
I have not read this study in full so remember to keep a deal of skepticism with the results (it was an in vitro study looking at erythrocytes (red blood cells) that also relied on mathematical modeling to map NAC concentrations needed for glutathione production), but I’ll post the conclusion here to provide a bit of information on this study:
Although at high concentrations in the plasma, cystine is not an effective source of cysteine for GSH synthesis by RBCs. NAC reacts with cystine, reducing it to cysteine and producing NAC-NAC and NACcysteine. Analysis of the reaction scheme with a mathematical model demonstrated that, at the plasma concentrations of NAC achieved therapeutically, cysteine would be produced at a sufficiently high rate by reduction of cystine to support rates of synthesis that are sufficient to sustain normal GSH concentrations even in oxidatively stressed RBCs
Yes, so not only is it possible that NAC may become deacylated and increase intracellular levels of L-cysteine, but that L-cysteine restoration may actually stem from reduction of plasma cystine because of NAC. Just even more confusion added to the whole NAC/L-cysteine issue, but that’s what happens when you go down the rabbit hole!
A theoretical scheme is provided in the Whillier, et. al. study:

HOWEVER, remember that Pedre, et. al. has provided rebuttal to NAC as a reducing agent. The supposed mucolytic activity of NAC requires that it works as a strong reducing agent, to which Pedre, et. al. has provided some pushback:
In summary, like other small monothiols, NAC clearly has the ability to directly reduce disulfide bonds in proteins or other molecules. However, the low reactivity and correspondingly slow kinetics argue against a widespread and general role as an extracellular disulfide reductant, limiting this mechanism to situations where the NAC concentration is at least reaching into the mM range. Also, the related idea that NAC taken up by cells (to be addressed below), is directly contributing to the reduction of intracellular disulfide bonds appears very unlikely. As will be discussed later, uptake of NAC into cells is rather slow and the disulfide reduction rate afforded by NAC is negligible when compared to the highly efficient enzymatic reducing systems (Nagy, 2013).
This sort of confuses the matter even further, but keep in mind this explanation relates to the mucolytic effects of NAC and may not necessarily apply to the scenario specific to cystine. In fact, further down we’ll notice that this slow reduction reaction may actually provide a benefit to NAC supplementation.
NAC safer than L-cysteine?
Pedre, et. al. does provides probably one of the most critical explanations as to why NAC may be more appropriate than L-cysteine alone, and that is toxicity:
Beyond the basic chemistry, there is another, far more critical argument in favor of NAC, namely its well-documented safety. NAC turned out to be safe in adults and children, even at very high doses, and studies in mice and rats show that toxic effects are only observed at very high dosages, above 6 g/kg when given orally and above 2 g/kg when injected intravenously (Bonanomi & Gazzaniga, 1980). For Cys, the situation looks very different. It has long been known that Cys can be toxic when administered at supraphysiological levels. Cys overdosing (oral LD50 ~6 g/kg in rats (European Chemicals Agency, 2021) can have severe pathophysiological consequences.
[…]
These and other observations have led to the notion that high concentrations of Cys are potentially toxic. It is interesting to note that normal intracellular Cys concentrations are lower than those of other amino acids (Bergström, Fürst, Norée, & Vinnars, 1974; Piez & Eagle, 1958; Soley & Alemany, 1980) and at the same time appear to be tightly regulated.
Measures of elevated blood cysteine may be indicative of metabolic and cardiovascular disease due to its role in producing oxidative damage4. However, care must be taken in understanding that the presence of cysteine may not be the cause of metabolic disease but a consequence of it.
Interestingly, Pedre, et. al. provides another argument in that L-cysteine may also be responsible for the production of hydrogen sulfide (H2S), a highly toxic agent which may also explain the cytotoxic effects of L-cysteine.
Several enzymatic pathways are responsible for the production of H2S from L-cysteine via desulfuration (removal of the sulfur). However, note that the direct association between H2S and L-cysteine cytotoxicity has not been fully elucidated:
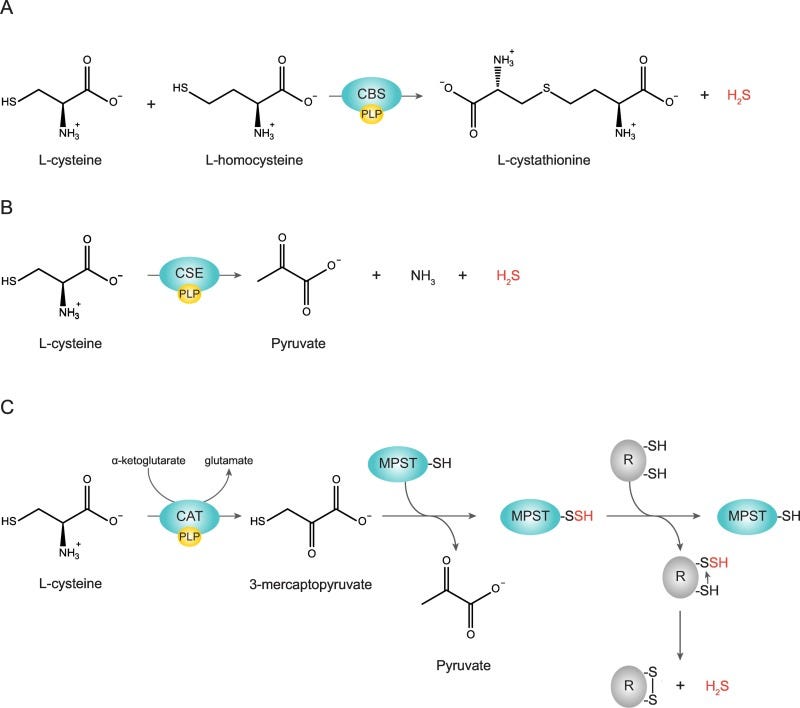
This would also raise questions as to whether these issues would be intrinsic to NAC as well. NAC is, after all, responsible for replenishing GSH via L-cysteine.
Overall, these ideas are rather interesting. When you look up labels for NAC supplements you’ll notice that they are labeled for “immune support” or as a “free-radical scavenger”. However, L-cysteine supplements are labeled for “structural support”. What structural support? Disulfide bonds are important for hair, skin, and nails so it’s likely where these labels are going (if you tend to have curly hair, it’s likely many of the proteins in your hair are full of disulfide bridges).
Although we shouldn’t look to supplement labels for contextual information, it’s pretty interesting that these two compounds are labeled differently, and maybe that might mean something for how these two supplements work.

Does NAC bypass L-cysteine toxicity?
As such, it appears that NAC would be a more attractive supplement for the mere fact that it may show less toxicity than direct supplementation with L-cysteine.
And this point is made further by Pedre, et. al. Remember that NAC must first be deacetylated in order to become L-cysteine. This reaction itself is rather slow, meaning that the conversion is a slow, controlled process rather than a large, immediate load at one time:
Most likely, the answer is connected to the pharmacokinetic bottlenecks that are imposed by the N-acetyl group. It appears that NAC releases Cys slowly, thus preventing acute exposure of tissues to high (i.e. supraphysiological) concentrations of Cys, and thus of H2S. Although we could not find studies directly comparing the pharmacokinetics of Cys and NAC, the idea of NAC being a ‘slow donor’ of Cys is well supported by cell-based studies which show a much slower catabolism of NAC relative to Cys (Banks & Stipanuk, 1994; Raftos, Whillier, Chapman, & Kuchel, 2007).
As indicated above, keep in mind that this framework is rather hypothetical. In fact, much of the work here may be considered hypothetical with a few supporting evidence from studies that have not been fully substantiated.
In addition, NAC can undergo 2 possible pathways of cellular entryway. One is that NAC can pass into the cell. This process is slow due to the N-acetyl group which hinders both active and passive transport of the molecule into the cell. After NAC enters the cell it becomes deacetylated by the enzyme Aminoacylase 1, which again is a slow process5. Together, these two processes lead to slow uptake and utilization of NAC, as indicated by the slow increase in serum concentration of NAC:
The slow cellular uptake and deacetylation of NAC explains the time needed to reach peak concentration (≥0.5 h) (Holdiness, 1991) and the mean residence time in the bloodstream (≥2 h) (Borgström, Kågedal, & Paulsen, 1986; Olsson et al., 1988) following oral administration.
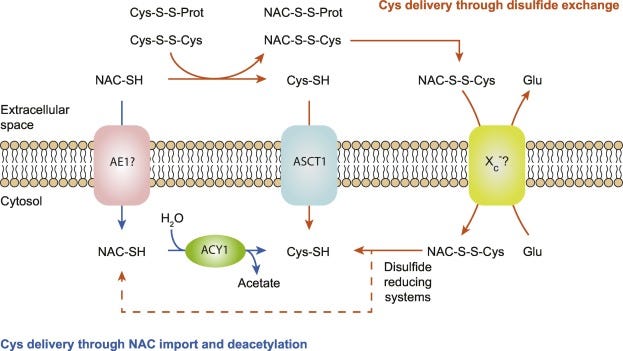
The other pathway is one argued in the Whillier, et. al. study, and that is NAC as a reducing agent for plasma cystine which may lead to uptake of reduced L-cysteine by cells (Pedre, et. al.):
The second mechanism of NAC-to-Cys conversion is indirect and involves disulfide exchange between NAC and disulfide-linked Cys in the extracellular space (mainly Cys-S-S-Cys and Cys-S-S-Protein), generating corresponding NAC mixed disulfides (NAC-S-S-Cys and NAC-S-S-Protein) and releasing reduced Cys that can be taken up by surrounding cells, presumably by the ASCT1 transporter mentioned above (Radtke et al., 2012; Raftos et al., 2007) (Fig. 5).
This process is also slow, including both the reduction of cystine as well as the transport of Cys-S-S-NAC dimers. Again, this may actually be a benefit as it dampens the immediate release of L-cysteine.
Overall, NAC may be considered more beneficial by virtue of being a more slow, controlled release of L-cysteine that may attenuate much of the oxidative damage that may come with high levels of L-cysteine:
In summary, it seems that NAC delivers Cys at such a slow and (presumably) steady pace that it avoids the toxic effects that have been associated with corresponding dosages of unmodified Cys. In other words, N-acetylation of Cys slows down the delivery of Cys, making NAC a Cys-prodrug that feeds cells with a trickle of Cys over a prolonged period of time.

What about NAC over GSH?
We’ve covered (rather exhaustively) why NAC may be considered better than L-cysteine as a supplement. Now, we may want to look at GSH.
First off, I’ll warn that this review will be spotty due to lack of research in the literature compared to NAC, so we will make do with what we have.
Anyways, compared to NAC why not go all the way, cut out the middle man and just go full throttle into the active antioxidant in question?
One main concern with GSH is that it is a tripeptide (3 amino acids), making it susceptible to digestion by enzymes or through other reactions.
One review by Minich, D. M., & Brown, B. I.6 takes a look at GSH literature and provides some conflicting evidence:
It would seem to be most efficient to administer oral glutathione as a preformed compound to override the effects of potentially inefficient SNPs and related enzymes. However, there has been some debate regarding whether glutathione given orally would be degraded by digestive peptidases [37,38]. In further support of this theory, some studies [39,40,41] have shown no change in glutathione levels or in parameters of oxidative stress despite acute [40,41] or chronic (four weeks) [39] oral glutathione supplementation.
There is also some evidence to the contrary. One six-month, randomized, double-blinded, placebo-controlled trial [42] found that taking oral glutathione at either 250 or 1000 mg/day led to significant increases in the body stores of glutathione in 54 non-smoking adults in a dose-dependent manner. There was also a decrease in the markers for oxidative stress at six months as indicated through an improvement in the oxidized (GSSG) to reduced (GSH) glutathione ratio in whole blood, in conjunction with favorable increases in natural killer cell cytotoxicity.
While the data on providing oral glutathione are mixed and inconclusive, recent research suggests that when glutathione is administered in liposomal or sublingual forms it may be made more bioavailable and favorably impact systemic glutathione levels.
Similar remarks were made in an exercise review from Kerksick, C. & Willoughby, D.7 that looked at evidence of NAC and GSH within the context of exercise:
Supplementation with glutathione has been met with little success as the bioavailability of glutathione is low due its transient transport throughout the cellular network. At the current time, the bioavailability of glutathione is thought to be extremely poor due to the hydrolytic enzymes that break down the glutathione upon ingestion. Nevertheless, popularity for glutathione administration and supplementation is high due to its primary role to minimize the oxidative stress seen with exercise.
Liposomal GSH takes GSH and places it into a film of lipids which may decrease the metabolism of GSH into its constituent parts. Modern research into GSH appears to focus on this form of GSH, and some of the current studies utilizing liposomal GSH as a therapeutic agent seem promising8,9,10.
From this, it’s important to examine the delivery mechanism of GSH used in studies. It’s likely that sublingual and intravenous administration of GSH may bypass the digestive mechanisms, and the inclusion of liposomal forms may aid in the protection of this molecule as well. This is likely where much of the conflicting results are derived as liposomal GSH may be far more superior than standard oral GSH.
But does this mean that supplementation with GSH would be futile?
Not necessarily. Remember that GSH metabolism would lead to the 3 amino acid constituents- cysteine, glycine, and glutamic acid. Afterwards, cells can then uptake these precursors and resynthesize GSH to restore intracellular GSH levels.
Essentially, GSH may be broken down only to be remade once again- a continuous cycle of GSH recycling.
A pretty big hassle, but the body won’t necessarily know what it should do with some of the nutrients and macromolecules that enter into it, so metabolism and easy transport is a general method of distributing nutrients. There is the possibility that these precursors for GSH could be used for other biochemical pathways, and under times where enzymatic processes may be attenuated (either by cellular stress or drugs that may serve as inhibitors for GSH enzymes) reformation of GSH may not be possible or greatly hindered.
On the other hand, elevated intracellular levels of L-cysteine can overcome the rate-limiting step and can provide the necessary limiting reagent to drive the synthesis of GSH.
It’s worth noting that breakdown of extracellular GSH for uptake and resynthesis of GSH is not quite out of the ordinary for cells, with some evidence provided in a review by Zhang, H., Forman, H. J., & Choi, J.11.
Much of the metabolism of GSH first starts with the enzyme γ‐Glutamyl Transpeptidase12, an enzyme found on the membrane of cells which cleaves the bond between glutamic acid and cysteine leaving cysteinylglycine (the dipeptide comprised of cysteine and glycine). This dipeptide gets further broken down by other enzymes, and eventually the constituents amino acids are taken up by cells to reform GSH (Zhang, H., Forman, H. J., & Choi, J.):
GGT catalyzes the transfer of γ‐glutamyl moiety from GSH, GSH S‐conjugates, and other γ‐glutamyl compounds to acceptors such as amino acids, dipeptides, and H2O. The catalytic mechanism of GGT is well known (Taniguchi and Ikeda, 1998; Tate and Meister, 1981). As discussed previously, GGT plays a key role in γ‐glutamyl cycle (Fig. 1) in the de novo synthesis of GSH (Meister, 1974; Stark et al., 2003). Here, GGT breaks down extracellular GSH to generate γ‐glutamyl compounds and cysteinylglycine, which is further cleaved by membrane‐bound dipeptidases. The constituent amino acids are then taken up and used by cells for intracellular resynthesis of GSH. One of the amino acids that are thus supplied by GGT is cysteine, the limiting substrate for GSH biosynthesis. Importantly, cysteine is a preferred acceptor for glutamate‐amino acid conjugation reaction by GGT, and the product, γ‐glutamylcystine, can be transported inside the cells and, after reduction to γ‐GC, used directly for GSH biosynthesis (Anderson and Meister, 1983; Meister, 1984). This GSH salvage pathway bypasses the rate‐limiting reaction catalyzed by GCL in de novo synthesis of GSH (Griffith et al., 1981) and may play an important role in the maintenance of GSH in cells.
There’s also the fact that extracellular GSH levels tend to be elevated by pulmonary epithelium, and so extracellular GSH may be important for aiding in some of the symptoms associated with ARDS.
Overall, GSH supplementation may be considered rather futile unless provided in a form that can bypass digestion such as the more recent liposomal form. There are a few forms that appear to be available, although I can’t speak of the veracity of these supplements.
However, even if one were to use typical, non-liposomal GSH the constituents will still be there even if GSH is broken down. The body can still uptake the constituent amino acids and reform GSH within the intracellular space, and there’s evidence to suggest that this occurs as a method of maintaining GSH homeostasis. So not all hope is lost if one were to use regular GSH, but we’ll look at one last option for boosting L-cysteine and GSH levels, and that’s through doing things that our bodies and ancestors have been doing for millions of years: eating and making it ourselves.
Homegrown or Eating out: making and sourcing L-cysteine from foods
Supplements are usually considered to be a last-line resort so to speak, which should usually be used in instances where someone is heavily deficient in the given compound or vitamin. I supplement with Vitamin D3 because my family is Vitamin D3 deficient, and so for me that makes sense that I should take daily Vitamin D3.
Although vitamins and supplements are relatively cheap compared to the grander scheme of drugs and pharmaceuticals, we may wonder if we should be taking capsules when we can just eat foods to obtain the necessary nutrients- our ancestors have always done it this way.
Sourcing L-cysteine from foods
Compared to other nutrients, the sourcing of amino acids is probably one of the easiest things we can do. Much like carbohydrates, amino acids serve as one of the pivotal building blocks of all life, so by eating a variety of foods we’re bound to come across L-cysteine and GSH at some point.
L-cysteine is considered a semiessential amino acid since our body can produce it in low amounts, however some researchers have considered L-cysteine to be conditionally essential because of the significance of sulfur compounds on health, especially for formation of GSH.
It’s a bit difficult to source literature detailing foods high in L-cysteine, but the general consensus may not be one that would make my vegetarian/vegan readers all too happy.
Most dietary sources of L-cysteine is derived from animal products such as meat, eggs, and cheese.

I can’t speak to the veracity of this chart specifically, but meat appears to be a consistent food source mentioned.
Some research may suggest that reduced protein intake may be correlated with reduced GSH production (Minich, D. M., & Brown, B. I.):
Since the precursors and foundation of glutathione are amino acids, intake of dietary protein may influence the amino acid pool from which to draw to synthesize glutathione. Changes in protein consumption [69], including reducing protein levels but remaining within safe levels, may alter plasma glutathione synthesis levels contributing to a reduction in antioxidant capacity. In this study, the researchers found that while individuals were able to recover from a reduction in protein (that remained above the lowest amount considered safe) in terms of nitrogen balance, it took longer for the functional changes in glutathione levels to equilibrate. Urinary excretion of 5-L-oxoproline was suggested as a marker to track glutathione kinetics, particularly the availability of glycine.
So dietary intake of protein may be pivotal to GSH production. Maybe Danny DeVito was onto something.
L-cysteine supplementation research has been quite extensive, with one review coming from Clemente Plaza, et. al.13

However, research into dietary L-cysteine and supplementation has provided mixed reviews, indicating the importance of balancing the redox potential of L-cysteine and cysteine conjugates- will L-cysteine form antioxidant conjugates, or will some of these sulfur conjugates cause oxidative damage (Clemente Plaza, et. al.):
Despite the high volume of evidence describing positive impacts of l-Cys on human health, it is worth noting that the number of works describing no effects or negative effects is also significant [20,77,78]. Negative effects of l-Cys derivatives on human health have been reported [79,80]. Thus, although most of the studies on l-Cys highlight its role in the homeostasis of redox status, some studies suggest that redox modulation is not involved during l-Cys actions and that l-Cys might act as a competitive antagonist of GABAA ρ1 receptors for instance [20]. Also, some in vivo studies have shown that several S-conjugates are nephrotoxic and that the toxicity is associated with β-lyase-dependent bioactivation [79]; in other cases, toxic effects of l-Cys on the nervous system have also been reported (oxidized l-Cys derivatives or compounds such as cysteine alpha-carbamate caused neuronal degeneration) [78].
L-cysteine enrichment seems to have reached a field that all of us strive for but probably aren’t getting enough of, and that’s fitness through the use of whey protein. Some researchers have examined whether whey protein would be a good source of L-cysteine and GSH14.
Minich, D. M., & Brown, B. I. provides additional context:
Although it is not necessary for most people to supplement with protein to meet their daily requirement, one potential beneficial source when additional protein is necessary is whey protein, likely due to its higher cysteine content [70]. In a small study (n = 18) of healthy individuals, whey protein supplementation at a dose of 15, 30, or 45 g/day for 14-days resulted in a dose dependent increase in lymphocyte glutathione levels with the 45 g/day dose increasing lymphocyte glutathione by 24% [71]. In another small randomized control study on cancer patients (n = 23) [72], consuming 40 g of whey protein isolates in addition to zinc and selenium increased the glutathione levels (11.7%) as well as functional immune markers, including an increase of 4.8% in their immunoglobulin G levels compared with the control group (n = 19). Additionally, a small study of patients with Parkinson’s disease [70] found that supplementing with whey protein compared with soy protein led to a significant increase in the glutathione levels in the blood and the GSH/GSSG ratio, although there was no significant impact on the clinical markers of the disease.
This has led some people to consider enriching protein powder with L-cysteine to provide additional antioxidant benefits. However, protein powders have been known to have their protein levels doctors by the addition of inefficient amino acids, and so to the extent that protein powders would be a good source of L-cysteine would weigh (whey?) heavily upon the transparency of manufacturers.
If GSH is more to your liking you’ll actually be in luck. Plants utilize the antioxidant properties of GSH the same way that we do, and so many fruits and vegetables are rich in GSH in order to combat oxidation.
Again, cannot speak to the veracity of this chart (many of these foods may be rich in sulfur compounds; not necessarily GSH) but you’re likely to come across many other sources of GSH.
It’s important to note that older foods that appear discolored are visual signs that oxidation and free radical formation is likely occurring within these foods, and so keep that in mind when considering eating rotten fruits and vegetables- you may be eating more free radicals rather than free radical scavengers.
Is NAC really necessary?
As mentioned above L-cysteine is a semiessential amino acid because our body has pathways to produce it ourselves.
One example can be seen below from Minich, D. M., & Brown, B. I. This is one pathway used by our liver to produce both L-cysteine and GSH. Note all of the cofactors (in blue) needed for this pathway.

So if the ingredients and machinery are there to make GSH is supplementation really necessary?
I’ve mentioned this circumstance previously, but under times where cellular stress is high our cells may not be able to produce GSH. GSH depletion may be a consequence of our cells’ inability to replenish new GSH.
And there are plenty of maladies associated with GSH deficiency such as diabetes, obesity, cancer, and aging being a few.
What’s not noted in the list above is the significance of the gut microbiome, as many of our B vitamin cofactors come from our gut bacteria- reduced production due to gut dysbiosis is likely to affect cellular processes and thus GSH production.
So this creates a strange, paradoxical phenomenon. By virtue of these diseases and their potential oxidative damage GSH may be depleted in an attempt to attenuate the oxidative stress. However, a sick body itself may not be able to produce the necessary components due to reduced cell function. Most studies may not take into account the cause/effect relationship between chronic illness and GSH depletion, therefore missing out on some important subtleties.
When it comes to aging, there’s clear evidence of GSH deficiency owing to the aging body. Gut dysbiosis, reduced nutrient absorption, lower cellular mechanisms, and reduced digestion capacity caused by senescence all factor into the deficiency seen among the elderly. The same goes for many other nutrients, and so in such a population it may be worthwhile to supplement with GSH or NAC because the body no longer has the capacity to do so on its own. It’s why much of the research into GSH has looked at its role in neurodegenerative disease.
But that really hammers down an important point here. In our discussion we’ve explained all of the possible benefits of supplements when weighed with the possible harms, but we never really consider the role our body plays in producing some of these nutrients. When our body fails to produce these necessary compounds in acute disease, it may be worth it to try supplementing with exogenous substances.
However, if we place ourselves into a chronic state of illness and disease, therefore damaging our body’s own capacity to make its own nutrients, then we may need to reconsider exactly what purpose supplements may serve. In such a state, supplements may be nothing more than a bandage placed over the disease. It ironically serves as a method of reducing the symptoms rather than doing something to deal with the actual disease in question.
Rather than supplement, we may want to examine why our bodies are failing to make the necessary nutrients in the first place- and that goes the same for my family’s reduced vitamin D3 levels and much of the W.E.I.R.D.15 world.
Are there dietary reasons for why we are missing out on vital nutrients, owing to modern eating habits that usually follow the mantra, “brown food tastes good”, and therefore sacrificing nutrient-density over flavor and sugar-laden, processed commodities? Or maybe it’s lack of sunlight and the outdoor environment that is causing our deficiencies.
Obesity and diabetes are a consequence of modernity, and if GSH deficiency occurs in these populations wouldn’t we want to consider whether losing weight and proper management of diabetes would restore much of the cellular processes required to produce GSH ourselves rather than relying on supplements that don’t get to the root of the problem?
I’ve been thinking about this issue more after reader Zade raised interesting concerns about Melatonin in my post from June. In it she (or he?) mentioned that melatonin is considered a neurohormone in Europe and therefore falls under different regulations, and linking to this interesting study from Guardiola-Lemaître B.16 about possible toxicity in regards to Melatonin.
I haven't read it fully and only skimmed a bit, but what stood out is that much of the concerns over Melatonin aren't necessarily rooted in its toxicity or disruption of the circadian rhythm, but that supplementing with Melatonin may be done in response to altered sleep-wake cycles as seen in those with night-shift work. The review also mentions alterations in Melatonin production as a consequence of late-night eating, and so in essence exogenous Melatonin may be a correction for behaviors and lifestyle choices than run counter to what our bodies would normally want.
I haven’t taken Melatonin in a while and the new form I bought has given me really strange dreams. But this revelation sort of puts into perspective why I would personally want to use Melatonin.
Is it for good sleep? I normally get that already so that’s not quite the issue. Maybe there are people such as the elderly that can’t quite produce Melatonin anymore and needs to supplement. Or maybe there are people whose lives don’t quite allow for regular sleep schedules and Melatonin may help put them to sleep.
It’s the last one that we should take notice of. If we are using Melatonin as a compensation for poor behaviors wouldn’t it be far more beneficial to attempt to correct those behaviors to the best of our ability before turning to supplements?
And the same goes for NAC supplementation- wouldn’t it be far more better to understand exactly what is going on and why our bodies may not be able to replenish GSH before we find methods of supplementing that may gloss over these issues? Remember that these goes for things that we have control over, and not for things such as diseases of unknown etiology or things with no known viable treatments.
We usually discuss our relationship with food and how that affects our bodies, but we should probably be doing the same when it comes to supplements. The whole purpose for me writing about drugs and supplements is so that people gain a better understanding of their relationship to the things they put in their bodies.
We’ve made plenty of righteous remarks about vaccines, yet we should make sure to do the same with supplements rather than taking things for the sake of being told to do so.
As we enter into the weekend, consider taking some time and rummaging through your medicine cabinet (or wherever you keep supplements). Take a look at what you are taking. See if you have good, valid reasons for taking the supplements you are, or maybe see if you are taking them because word-of-mouth or secondhand comments have told you these were good. Are you taking Melatonin even though you get good sleep? Do you take Vitamin D3 even though you are outside most of the day? Have some Quercetin on-hand? Well so does fruits and vegetables, so why not try with whole foods instead?
I should take care to note that this SHOULD NOT be used as medical advice, but to argue that we should gain some perspective and understanding for what we take.
As to NAC, I mostly bought it because I was told I couldn’t have it. But given these findings I’m going to consider whether I find it necessary to take on a daily basis.
This post has gone far longer than I thought it would, and it’s nowhere near finished scratching the surface.
But hopefully it gives you an understanding of NAC, and whether we may find it necessary to supplement.
If you enjoyed this post and other works please consider supporting me through a paid Substack subscription or through my Ko-fi. Any bit helps, and it encourages independent creators and journalists outside the mainstream.

Pedre, B., Barayeu, U., Ezeriņa, D., & Dick, T. P. (2021). The mechanism of action of N-acetylcysteine (NAC): The emerging role of H2S and sulfane sulfur species. Pharmacology & therapeutics, 228, 107916. https://doi.org/10.1016/j.pharmthera.2021.107916
Those interested in the chemical reasoning for this reduced oxidation should refer to the Pedre, et. al. paragraph after Table 1, which provides an explanation due to the pKa of the thiol group in relation to the ammonium.
Whillier, S., Raftos, J. E., Chapman, B., & Kuchel, P. W. (2009). Role of N-acetylcysteine and cystine in glutathione synthesis in human erythrocytes. Redox report : communications in free radical research, 14(3), 115–124. https://doi.org/10.1179/135100009X392539
Mohorko, N., Petelin, A., Jurdana, M., Biolo, G., & Jenko-Pražnikar, Z. (2015). Elevated serum levels of cysteine and tyrosine: early biomarkers in asymptomatic adults at increased risk of developing metabolic syndrome. BioMed research international, 2015, 418681. https://doi.org/10.1155/2015/418681
As noted by Pedre, et. al. it would appear that the uptake of NAC would be considered the rate-limiting step and not the deacetylation by the enzyme.
Minich, D. M., & Brown, B. I. (2019). A Review of Dietary (Phyto)Nutrients for Glutathione Support. Nutrients, 11(9), 2073. https://doi.org/10.3390/nu11092073
Chad Kerksick & Darryn Willoughby (2005) The Antioxidant Role of Glutathione and N-Acetyl-Cysteine Supplements and Exercise-Induced Oxidative Stress, Journal of the International Society of Sports Nutrition, 2:2, DOI: 10.1186/1550-2783-2-2-38
To, K., Cao, R., Yegiazaryan, A., Owens, J., Nguyen, T., Sasaninia, K., Vaughn, C., Singh, M., Truong, E., Medina, A., Avitia, E., Villegas, J., Pham, C., Sathananthan, A., & Venketaraman, V. (2021). Effects of Oral Liposomal Glutathione in Altering the Immune Responses Against Mycobacterium tuberculosis and the Mycobacterium bovis BCG Strain in Individuals With Type 2 Diabetes. Frontiers in cellular and infection microbiology, 11, 657775. https://doi.org/10.3389/fcimb.2021.657775
Sinha, R., Sinha, I., Calcagnotto, A., Trushin, N., Haley, J. S., Schell, T. D., & Richie, J. P., Jr (2018). Oral supplementation with liposomal glutathione elevates body stores of glutathione and markers of immune function. European journal of clinical nutrition, 72(1), 105–111. https://doi.org/10.1038/ejcn.2017.132
Kachour, N., Beever, A., Owens, J., Cao, R., Kolloli, A., Kumar, R., Sasaninia, K., Vaughn, C., Singh, M., Truong, E., Khatchadourian, C., Sisliyan, C., Zakery, K., Khamas, W., Subbian, S., & Venketaraman, V. (2022). Liposomal Glutathione Helps to Mitigate Mycobacterium tuberculosis Infection in the Lungs. Antioxidants (Basel, Switzerland), 11(4), 673. https://doi.org/10.3390/antiox11040673
Zhang, H., Forman, H. J., & Choi, J. (2005). Gamma-glutamyl transpeptidase in glutathione biosynthesis. Methods in enzymology, 401, 468–483. https://doi.org/10.1016/S0076-6879(05)01028-1
Clemente Plaza, N., Reig García-Galbis, M., & Martínez-Espinosa, R. M. (2018). Effects of the Usage of l-Cysteine (l-Cys) on Human Health. Molecules (Basel, Switzerland), 23(3), 575. https://doi.org/10.3390/molecules23030575
Kent, K. D., Harper, W. J., & Bomser, J. A. (2003). Effect of whey protein isolate on intracellular glutathione and oxidant-induced cell death in human prostate epithelial cells. Toxicology in vitro : an international journal published in association with BIBRA, 17(1), 27–33. https://doi.org/10.1016/s0887-2333(02)00119-4
Western, Educated, Industrialized, Rich, And Democratic
Guardiola-Lemaître B. (1997). Toxicology of melatonin. Journal of biological rhythms, 12(6), 697–706. https://doi.org/10.1177/074873049701200627










Love, love, love this post!!! It's awesome to have someone go through these studies and explain the supplements better- I'm so full of gratitude!
Really interesting article with plenty of food for thought. Speaking of food, do you think most of the foods to which we have access are truly high quality, containing all the good things we assume they do? I have heard this might not be the case, even with organic produce, due to soil depletion, etc. Also, I do think aging is an important consideration. I am closer to 60 than 50 now, not able to run the distances I used to and therefore, must eat less to maintain healthy weight. I eat well, but not much. Supplementation seems to make more sense for me now, than it would have 20 years ago. But I do agree. Natural sources would be the way to go, if possible.