It's not novel to SARS-COV2
Loss of taste and smell have been reported in the literature following other ARIs, so why have these gone unnoticed or understudied?
In one of my prior posts Peter Nayland Kust of All Facts Matter asked a question about looking into “Long Flu” for context in regards to Long COVID. This article is a brief assessment for post-viral syndromes, in particular olfactory dysfunction, following other viruses aside from SARS-COV2.
Probably one of the worst things to come from this pandemic was the labeling of SARS-COV2 as being “novel”.
Inherently, the use of novel to described SARS-COV2 was done with the intent of suggesting that the virus itself was new to us humans. At the same time the use of novel has also led any symptom and pathologies related to SARS-COV2 to also be labeled “novel”.
As Brian Mowrey likes to comment we always need a comparator, or put into his words, “compared to what?” That is to say, it becomes easy to consider all things SARS-COV2 related to be novel to SARS-COV2 if you don’t look elsewhere.
This has created serious problems, especially within the realm of Long COVID. Because post-viral syndrome has been underreported- if reported at all within the literature- the argued novelty of this syndrome can be easily be rebutted by suggesting that Long COVID doesn’t exist, and relying on data that tends to poorly categorize Long COVID. The irony in this is that if Long COVID can’t be accurately categorized then the same may be happening for post-viral syndrome as a whole, leading to why this pathology may be infrequently studied or reported.
Again, many of these claims rebutting Long COVID tend to come from those who don’t scour the internet to find those comparators, and given that Long COVID symptoms overlap with HHV reactivation, which appears to appear frequently following SARS-COV2 infection then it tells us that there is an underlying mechanism that is being overlooked.
So the question to be asked is not whether Long COVID is novel to SARS-COV2, but to ask what evidence there is to suggest that this may be a relatively common feature of other upper respiratory infections.
Interestingly a recent study published in The Lancet1 asked a similar question:
This spotlight on the long-term effects of SARS-CoV-2 infection leads to the question of whether there are post-acute sequelae of other acute respiratory infections (ARIs) in the community, including those that present with mild initial symptoms, and how these sequelae compare with long COVID. Importantly, given the non-specific nature of symptoms such as fatigue, any comparison needs to take into account the symptom prevalence in a contemporaneous, uninfected population—particularly given the background of a disruptive pandemic, with increased stressors, limitations on movements, and elevated levels of population fatigue.12
The study enrolled people who reported previous infections, either SARS-COV2 or other acute respiratory infections (ARI) and asked them questionnaires relating to their symptoms.
The study enrollment started May 1, 2020 up until October 6, 2021. However, included participants were those who responded to the January 2021 follow-up questionnaire who were not vaccinated with the COVID vaccine, and so no vaccinated individuals were included in the study.
SARS-COV2 infection was verified in a similar way as other studies by PCR or antigen-confirmed testing. For those who tested positive with an antigen test but could not recall an infection date the date of symptom onset was used.
For those with other ARIs participants either had to have had a diagnosis from a general practitioner or hospital of other infections or symptoms not caused by SARS-COV2. However, it appears that self-reported symptoms along with a negative SARS-COV2 test were also allowed:
We defined a previous non-COVID-19 ARI as self-report of a general practitioner or hospital diagnosis of pneumonia, influenza, bronchitis, tonsillitis, pharyngitis, ear infection, common cold, or other upper or lower respiratory infection not caused by SARS-CoV-2; or self-report of a symptom-defined ARI accompanied by a negative SARS-CoV-2 swab test (lateral flow or RT-PCR).
It appears that most non-COVID ARI infections were self-reported, which is to be expected given that there were far fewer hospitalizations for infection within the non-COVID-19 ARI group relative to the COVID group.
Participants who reported both a previous SARS-COV2 infection as well as a previous non-COVID ARI were excluded.
Note that there are a few discrepancies with this study, such that there is no hard-defined 12 week post-infection period, and so in some portions of the study where Long COVID is mentioned bear in mind that this may include people with more acute symptoms. I can’t find evidence of whether participants were excluded from the Figure 1 analysis if below the 12 week mark since infection, although the questionnaire reports around 87% of SARS-COV2 participants were infected more than 12 weeks prior. The issue is worse with the non-COVID ARI group which appears to report around 41% of participants being 12 weeks post their ARI infection, meaning a large portion of this cohort may not be comparable to Long COVID participants given the shorter timeframe since infection.
With that word of caution in mind, the important thing to look at from this study is Figure 1. Figure 1A stratified previously SARS-COV2 positive participants based upon their ongoing symptom severity, with the “most severe” group being comprised of those who reported marked increases in all 16 symptoms following a COVID infection. This group also appears to be comprised largely of people with severe infection and requiring hospitalization:

But the interesting figure worth looking at is Figure 1B, which compared the groups with the most severe symptoms from both the SARS-COV2 group as well as the non-COVID ARI groups. A control group of those who were not infected was also included for comparison.
Interestingly, when looking at symptom profiles between the two infection groups symptom scores were comparable aside from SARS-COV2 which reported higher rates of memory problems and loss of taste/smell:
In this visual comparison, participants with non-COVID-19 ARI or SARS-CoV-2 infection showed a higher probability of reporting almost all symptoms than participants with no infection, particularly cognitive problems (Fig. 1B). However, symptom profiles seem to differ by ARI among those most severely affected: participants with SARS-CoV-2 infection showed a higher probability of reporting memory problems, difficulty concentrating, unusual racing of the heart, unusual sweating, hair loss, and problems with sense of taste or smell compared with non-COVID-19 ARI (Fig. 1B).
Now, there are a lot of limitations to this study as there is a ton of ambiguity for the non-COVID ARI group. Differences in hospitalization rates as well as age (the non-infected group leaning older) should also be taken into account given that there were higher rates of SARS-COV2 hospitalization relative to non-COVID ARI participants. Note that the study also doesn’t include any measure of symptom recovery, but rather used a specific timepoint, likely the January 2021 follow-up questionnaire, as the only time which participants were evaluated. The fact that this is an online questionnaire also creates issues with recall bias, with some non-COVID ARI participants attributing their symptoms to COVID even though they tested negative. Some SARS-COV2 participants also didn’t appear to associate their ongoing symptoms to SARS-COV2, meaning that people may be misattributing where their ongoing symptoms may come from.
That being said, this study at least notes that people who have experienced other respiratory infections may also experience ongoing symptoms in the weeks or months afterwards, which is something that may have been previously overlooked.
The authors conclude their study with the following comments, suggesting that post-viral syndrome or symptoms following ARI may be underestimated:
The COVID-19 pandemic has cast a much-needed spotlight on post-acute infection syndromes, highlighting the need for improved understanding, diagnosis, and treatment of these conditions. While the high symptom burden we observed in participants with previous SARS-CoV-2 infection illustrates the extensive reach of long COVID, the similar burden observed among people with previous non-COVID-19 ARI suggests that the lasting impacts of these infections may be underestimated. As research into long COVID continues, we must take the opportunity to investigate and consider the post-acute burden of other ARIs, to ensure all people with post-acute sequelae can access the treatment and care they deserve.
It’s an important reminder that there are a lot of unknowns when it comes to other respiratory infections. It’s only through the studies being conducted over SARS-COV2 that this wide discrepancy in knowledge between SARS-COV2 and other viruses has become more noticeable. And so, it’s not necessarily that things are novel to SARS-COV2, but more that we lack the same number of studies for other viruses and bacteria, meaning that there’s likely to be areas under researched.
The prior study is more food for thought. I only came across it today when looking at things in my news feed.
The original intention of this post is to look at alterations in taste and smell in particular following other viral infections. As these seem to be common features among Long COVID sufferers, it would be worth finding comparators within the literature.
One of the earliest studies2 to examine reduced taste and smell following a viral infection occurred in 1975, in which researchers at the Taste and Smell Clinic at the NIH looked at patients who appeared to have postinfluenza-like hypogeusia (reduced taste) and hyposmia (reduced smell), or PIHH.
These patients began experiencing their symptoms following the winter and spring months where an infection of viral origin occurred, with those who were infected during the 1968-1969 winter and spring seasons argued to have been infected with the “Hong Kong Flu”, or H3N2 Influenza A strain.
Interestingly, although these patients referred to their influenza-like infections as “the worst they ever had”, these patients also didn’t appear to seek out treatment, and instead treated their infection with OTC medications.
However, these patients began experiencing reduced taste and smell which didn’t resolve, with some patients experiencing alterations in taste (dysgeusia3) and smell (dysosmia) which worsened during the recovery period.
Patients were subsequently referred to the NIH’s Smell and Taste Clinic due to exhausted diagnostic pathways of the patient’s physicians:
Patients were referred to our clinic by physicians who had exhausted all their knowledge of diagnosis or treatment of these distressing symptoms. Patients were referred from local family physicians, neurologists, otorhinolaryngologists, and psychiatrists.
The most noticeable alterations in these PIHH patients appeared to be the nose, where the development of PIHH was noted to be associated with drier nasal passages with reduced secretions.
Assessment by Henkin, et al. noted the following changes:
The most prominent consistent clinical feature of PIHH was in the nose where the tenacious, clear, occasionally white, mucous blanket normally found was not observed. The nasal mucous membranes were moist but neither shiny nor glistening. The membranes were often pale in color, but not similar to the pale, boggy bluish tinge commonly observed in allergic rhinitis or the reddish tinge seen in infectious rhinitis. Nasal membranes looked similar to those following the topical application of a vasoconstrictor agent. such as Neo-Synephrine. A second consistent feature was the patency of the nasal airway. This patency was such that the deeper structures of the nasal cavity were easily seen by anterior rhinoscopy without the use of vasoconstrictor agents. The opening of the glands of the nasal mucous mem-branes were not prominent. Membranes were not edematous, and there was little vascular congestion or turgor. Purulent secretions, crusting, or bleeding were not commonly observed. Two patients (2/87) had unilateral nasal polyps.
Nasal patency refers to how open the nose is, and it appears that for these patients the openings were wide and similar to that of those who use vasoconstrictor agents.
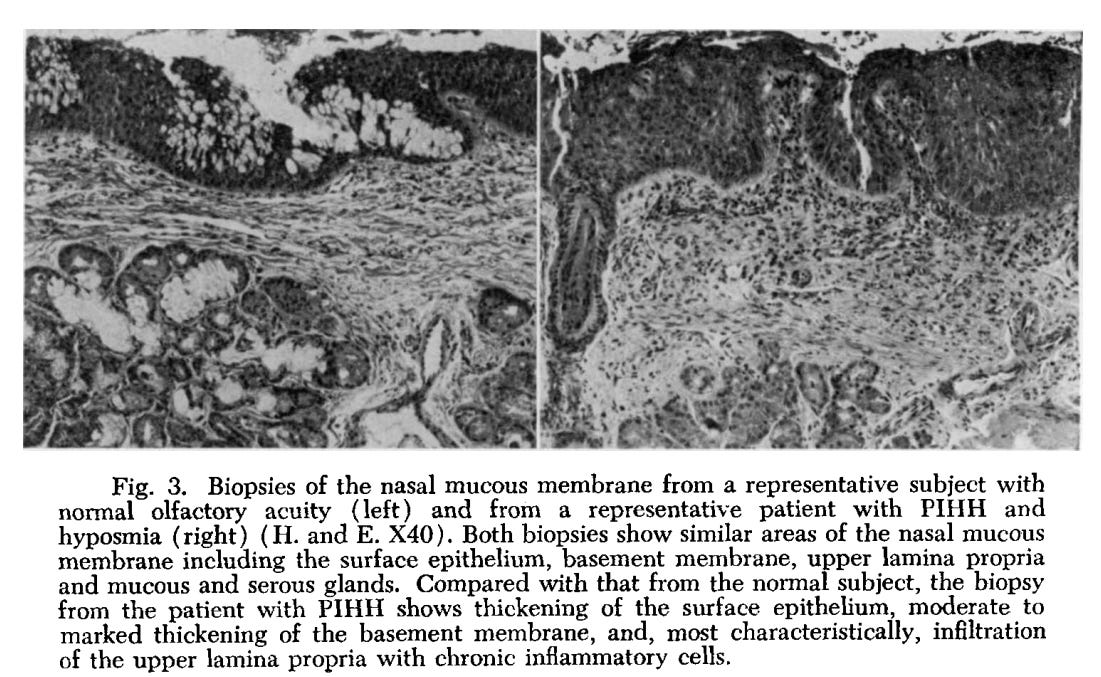
When a biopsy of a PIHH patient was compared to someone with normal olfaction one of the biggest distinctions was the presentation of inflammatory cells within the lamina propria, a layer of connective tissue found in most mucous membranes:
Representative biopsies from a subject with normal olfactory acuity and from a patient with PIHH and hyposmia are compared in Fig. 3. The biopsy from the patient with PIHH demonstrates thickening of the surface epithelium, with mild cellular atypia, moderate thickening of the basement membrane and, most characteristically, infiltration of the upper lamina propria with chronic inflammatory cells. These cells are mainly small lymphocytes, but some plasma cells were also observed. Relatively little, if any, inflammation was present in the submucosal glands. Significant sclerosis and fibrosis of the upper lamina propria was also commonly observed. Both serous and mucous glands were decreased in number, but serous gland were decreased to a much greater degree. Dysplasia of the ducts of some mucous glands was observed.
Although the viral etiology was assumed in this study, it’s clear that for these subset of patients post-infections led to clear nasal changes, with the downstream effects resulting in alterations in taste and smell.
More importantly, it suggests that evidence of post-viral syndrome relating to taste and smell is not inherently unique to SARS-COV2, but rather may be a feature of other respiratory infections as well.
In fact, aside from facial trauma, viral infections are considered one of the most likely causes for loss of smell and taste.
A 2006 review from Welge-Lüssen, A., & Wolfensberger, M. 4 noted that, given some of the studies published, that up to 40% of reported cases of olfactory dysfunction may be related to upper respiratory tract infections:
Epidemiological data in the literature vary considerably. According to a survey conducted by Damm et al. [8], approximately 11% of olfactory disorders treated at German, Austrian, and Swiss university hospitals are caused by URTI. In other studies, the prevalence of olfactory disorders caused by URTI is stated to be as high as 20–40% [3, 9–11]. These discrepant data are thought to be due to the variable patient populations studied. While the survey by Damm et al. [8] focused on general ear, nose, and throat clinics, other studies were conducted in specialized smell and taste centers that would be expected to see a different patient population. Women are more often affected than men [12–14], and this disorder typically occurs between the fourth and eighth decade of life [14, 15].
Remember that these values are wholly dependent upon the populations examined, how olfactory dysfunction was categorized, and how olfactory dysfunction was related to prior infection.
Nonetheless, it adds to the fact that, in time periods prior to the onset of SARS-COV2, there does appear to be some evidence of olfactory dysfunction following even common cold and flu seasons.
There are several other studies out there, although I’ll limit my remarks for now. What’s interesting is that the COVID pandemic has at least brought some attention to the idea that olfactory dysfunction and post-viral syndrome may have been grossly overlooked for decades, with renewed interest and several studies coming out only because of the pandemic, such as a systematic review from Lee, et al.5 as well as a small study from Passali, et al.6 which noted olfactory dysfunction including anosmia in some patients one month after a common cold infection.
Reasons for these changes in taste and smell appear to similar to those proposed for SARS-COV2-related anosmia, to the extent that either death of epithelial cells, inflammation of the nose, or direct damage to olfactory tissues have been proposed.
Lee, et al. provides this overview based on the animal and human studies reviewed (PVOD refers to post-viral olfactory dysfunction):
Our systematic review revealed that PVOD is attributable to disruption at many levels of the olfactory pathway by the cumulative effects of direct cell damage, inflammation, and cytokine effects. Most animal studies demonstrated that the numbers of ORNs are reduced in the OE and OB, which is likely the major cause of olfactory disruption following most types of viral infections. Importantly, our review also highlighted the fact that targets of virus-mediated cellular disruption in the olfactory system are viral substrain dependent. This has significant implications and emphasizes the need to study and direct patient care in a highly virus-specific manner following PVOD. Our review also found that human studies consistently suggest that both peripheral and central olfactory structures are negatively impacted in relation to structural integrity, volume, or metabolism. Many important studies in humans have shown putative viral agents in PVOD. For instance, high levels of parainfluenza virus 3 have been detected in patients with PVOD. 54 However, direct demonstration of specific pathophysiological changes within the olfactory system following viral subtype insults in humans has yet to be accomplished.
Note: ORN refers to olfactory receptor neurons, OE for olfactory epithelium, and OB for olfactory bulb
The authors propose a “Two-Hit” hypothesis, such that cell damage from the initial infection may then follow with reduced plasticity and impaired recovery, which may result in persistent symptoms:
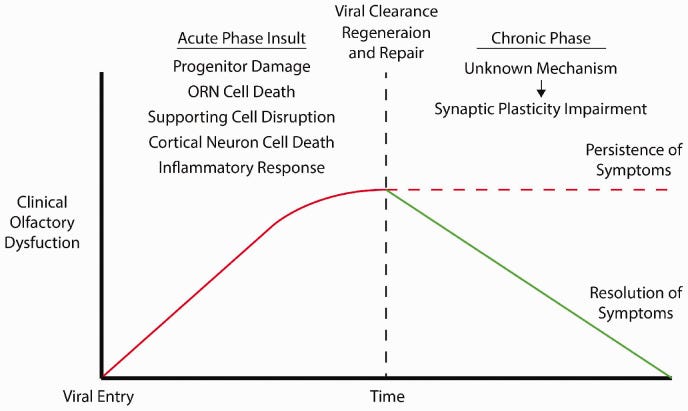
The intent of this post is not to dive deeper into the hypotheses for olfactory dysfunction, but again points out that there appears to be similar hypotheses between SARS-COV2 related olfactory dysfunction as well as dysfunction from even common colds and flus.
Overall, what this article hopefully notes is that these remarks about Long COVID, in particular those related to anosmia and other changes in taste and smell, are not unique to SARS-COV2 itself, but rather may be occur from commonly found respiratory viruses. Because of this care should be taken to not outright dismiss remarks that Long COVID doesn’t exist. More importantly, dismissal shouldn’t be done if the intent is to reduce any justification for lockdowns or masking. We can’t commit lies by omission.
Rather, this may just be more evidence that there has always been a risk of long-term effects from even common infections, but we’ve never stopped living in the meantime.. We should see who may be affected by Long COVID, and we should encourage research into seeing how many people may be affected by other forms of post-viral syndromes. But we don’t need to lock people down or force people to mask because this risk may have always been around.
Again, we can hold two ideas without them being contradictory.
Additional studies for those interested. Note that I haven’t read some of these, or some of these seem to be difficult to find.
Reden, et al.7: Recovery of olfactory function following closed head injury or infections of the upper respiratory tract
Yamagishi, et al.8: Olfactory mucosal findings and clinical course in patients with olfactory disorders following upper respiratory viral infection.
de Haro-Licer, et al.9: Long term serious olfactory loss in colds and/or flu.
The only version I came across was in Spanish so I couldn’t assess this one.
If you enjoyed this post and other works please consider supporting me through a paid Substack subscription or through my Ko-fi. Any bit helps, and it encourages independent creators and journalists such as myself to provide work outside of the mainstream narrative.

Vivaldi G, Pfeffer PE, Talaei M, Basera TJ, Shaheen SO, Martineau AR. (2023) Long-term symptom profiles after COVID-19 vs other acute respiratory infections: an analysis of data from the COVIDENCE UK study. eClinicalMedicine., doi: 10.1016/j.eclinm.2023.102251. https://www.thelancet.com/journals/eclinm/article/PIIS2589-5370(23)00428-5/fulltext
Henkin, R. I., Larson, A. L., & Powell, R. D. (1975). Hypogeusia, dysgeusia, hyposmia, and dysosmia following influenza-like infection. The Annals of otology, rhinology, and laryngology, 84(5 Pt 1), 672–682. https://doi.org/10.1177/000348947508400519
Note that dysgeusia is usually met with an unpleasant change in taste, although not all may experience unpleasantries. It’s likely that off tastes are more readily recognized than changes that may be more subtle.
Welge-Lüssen, A., & Wolfensberger, M. (2006). Olfactory disorders following upper respiratory tract infections. Advances in oto-rhino-laryngology, 63, 125–132. https://doi.org/10.1159/000093758
Lee, J. C., Nallani, R., Cass, L., Bhalla, V., Chiu, A. G., & Villwock, J. A. (2021). A Systematic Review of the Neuropathologic Findings of Post-Viral Olfactory Dysfunction: Implications and Novel Insight for the COVID-19 Pandemic. American journal of rhinology & allergy, 35(3), 323–333. https://doi.org/10.1177/1945892420957853
Passali, G. C., Passali, D., & Ciprandi, G. (2022). Postinfectious Olfactory Complaints: A Follow-up Study. International archives of otorhinolaryngology, 26(4), e657–e660. https://doi.org/10.1055/s-0042-1742761
Reden, J., Mueller, A., Mueller, C., Konstantinidis, I., Frasnelli, J., Landis, B. N., & Hummel, T. (2006). Recovery of olfactory function following closed head injury or infections of the upper respiratory tract. Archives of otolaryngology--head & neck surgery, 132(3), 265–269. https://doi.org/10.1001/archotol.132.3.265
Yamagishi, M., Fujiwara, M., & Nakamura, H. (1994). Olfactory mucosal findings and clinical course in patients with olfactory disorders following upper respiratory viral infection. Rhinology, 32(3), 113–118.
de Haro-Licer, J., Roura-Moreno, J., Vizitiu, A., González-Fernández, A., & González-Ares, J. A. (2013). Long term serious olfactory loss in colds and/or flu. Acta otorrinolaringologica espanola, 64(5), 331–338. https://doi.org/10.1016/j.otorri.2013.04.003





When I heard the virus presented as "novel" in early 2020, and one for which we had no natural immunity, I first laughed at the very idea and then started digging into just what kind of fraud was taking place. I'm a freelance consultant, and I had four months in 2020 with no billable time. There was a lot of digging to do.
I recall that, growing up in the 50s and 60s, we would sometimes lose our sense of taste when the nose stopped up, in addition to the obvious effect on smell. This was a normal thing, explained as perceived taste being a combination of the senses of taste and smell. When the nose was stopped up, taste was also affected. I've wondered if any of what is being reported now could be related to that.
I have a friend, however (not vaxxed) that went through three different rounds of something covid-like, where he lost taste and smell for an extended period of time. I talked with him about that experience, and what he described did not sound like what I recall from growing up.
LONG SARS ONE
EMMANUEL
https://twitter.com/ejustin46/status/1686009019549319168
LONG SARS1 !!!
EVERYTHING was already KNOWN in 2005!
"We have demonstrated widespread dissemination of the SARS virus in the immune cells of the blood, spleen, and lymph nodes, as well as in the epithelial cells of the lungs, trachea, ...
Multiple organ infection and the pathogenesis of SARS
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2213088/