It's gonna be a wet, hot, Incretin summer!
More pharmaceutical companies are coming out with newer, better, diabetes/weight-loss drugs just in time for beach season.
*Cover image from Goodrx
Summer’s just around the corner, but you still haven’t lost those lockdown 20’s or haven’t stuck with your not-so-new New Year’s Resolution to lose weight or get healthier, have you?
Do not fret, for Big Pharma has an answer for you!
In recent months several drugs such as Semaglutide (brand names Ozempic and Wegovy, among other names) have gained popularity with both Hollywood elites and the common suburbanite, leading to widespread shortages for those who the drug was initially intended for i.e. those with Type-II diabetes.
Many of these drugs work by mimicking incretin hormones, which are hormones released when eating that aid in the release of insulin and the shuttling of carbohydrates for storage.
In particular, most of these diabetic/weight-loss drugs are similar to one hormone peptide in particular called glucagon-like peptide 1 (GLP-1) and bind/activate GLP-1 receptors making them GLP-1 receptor agonists (GLP-1 RA1).
Much of this was covered in a previous post and won’t be rehashed:
What's going on with the Ozempic craze?
Modern Discontent is a reader-supported publication. To receive new posts and support my work, consider becoming a free or paid subscriber. By now, many readers have likely come across the new pharmac…
Eli Lilly & Tirzepatide
But as is the case for pharmaceutical manufacturers more will always be better. The newfound use of these GLP-1 RAs as weight-loss medications has increased the incentive to search for more of these incretin hormone simulacrums.
I covered one research endeavor in particular that appears to be combining various agents together, but just recently the pharmaceutical company Eli Lilly reported on much better results for their own weight-loss drug relative to Semaglutide.

Eli Lilly may have been swept aside as Ozempic and Wegovy saw all of the media attention last summer.
Apparently Eli Lilly’s drug Tirzepatide (brand name Mounjaro) received FDA approval in mid-May 2022 as a Type-II diabetes medication.
But within months, and likely due to the increasing popularity of these diabetes drugs as weight-loss medications, the FDA granted a fast-tracked designation for Tirzepatide as a weight-loss drug in October 2022, meaning that it could received fast-tracked approval based on the results from their SURMOUNT-2 clinical trial.
It was the results of this trial (SURMOUNT-2) which was just recently released, with some of the findings suggesting that those who were administered Tirzepatide had an average of 30 lbs of weight loss:
For the efficacy estimand, participants taking tirzepatide achieved average weight reductions of 13.4% (29.8 lb. or 13.5 kg) on 10 mg and 15.7% (34.4 lb. or 15.6 kg) on 15 mg compared to placebo (3.3%, 7.0 lb. or 3.2 kg). Additionally, 81.6% (10 mg) and 86.4% (15 mg) of people taking tirzepatide achieved at least 5% body weight reduction, the other co-primary endpoint, compared to 30.5% of those taking placebo.
Tirzepatide also met all key secondary objectives, which included reduction in A1C and other cardiometabolic parameters. 41.4% (10 mg) and 51.8% (15 mg) of people taking tirzepatide achieved at least 15% body weight reduction compared to 2.6% of those taking placebo. Reduction in A1C compared to placebo was similar to the SURPASS trials in adults with type 2 diabetes. Study participants had a mean baseline body weight of 222 lb. (100.7 kg) and baseline A1C of 8.0%.
With such results it’s likely that Tirzepatide will hit the market soon as possibly the only drug to be designated for use on-label as a weight-loss drug.
Tirzepatide Mechanism of Action
Part of these results appear to be related to Tirzepatide’s mechanism of action.
Rather than just acting as a GLP-1 receptor agonist like Semaglutide and other drugs already on the market, Tirzepatide acts on two different incretin receptors: the GLP-1 receptor as well as the glucose-dependent insulinotropic polypeptide receptor (GIPR).
The peptide hormone GIP acts as a similar incretin hormone as GLP-1, being released within the intestines to stimulate insulin production within the pancreas when someone eats food.
Tirzepatide appears to work as a stronger agonist for the GIPR, which makes sense given that it is modeled after the structure of native GIP.
The structure of Tirzepatide is shown below, with different modifications being highlighted (Chavda, et al.2):
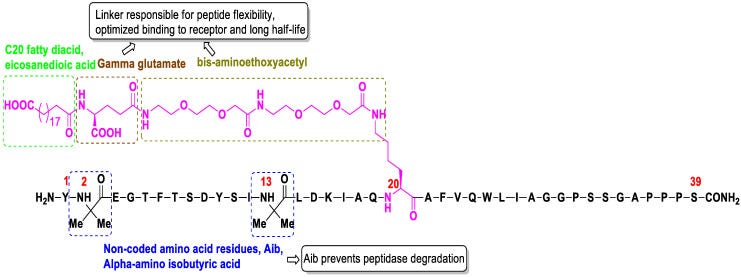
However this dual agonism for two incretin receptors suggests that Tirzepatide may have a stronger effect relative to GLP-1 RA drugs such as Semaglutide. This has led a few people to refer to Tirzepatide as a “twincretin” (Forzana, et al3):
A new molecule with a combined agonist action on both GPI and GLP1 receptors (“twincretin”) has been developed to take advantage of this synergistic effect: Tirzepatide (LY3298176) [26,27,28] is the first “twincretin”, a synthetic peptide composed of 39 amino acids based on the GIP native sequence [29,30], combining the dual agonism of GIP and GLP-1 receptors controlling glycemic blood level and reducing body weight (Figure 1). Tirzepatide has an affinity for the GIP receptor equal to that of native GIP and an affinity for the GLP-1 receptor ~5-fold weaker than that of native GLP-1 [31,32].
Research into GIP-mimicking molecules didn’t get off the ground much in past years, as it was assumed that such a drug may lead to GIP resistance. However, it appears that GIP resistance may possibly be reversed, and that the additional effects of GIP had renewed it as a molecule of interest (Fisman, E. Z., & Tenenbaum, A.4, emphasis mine):
GIP is the main incretin hormone in healthy people, causative of most the incretin effects but the insulin response after GIP secretion in T2DM is reduced [12]. It has been reported that there is no reduction in its secretion in patients with T2DM [13] but a nearly total loss of insulinotropic effect is observed, even at supraphysiological concentrations, implying the existence of GIP resistance [14]. Therefore, in the past GIP has been considered an unappealing therapeutic target for T2DM. This conception has been changing during recent years, since it has been reported that resistance to GIP can be reversed and its effectiveness restored by improving glycemic control [14]. A potential additional advantage of GIP is the protection against hypoglycemia since GIP infusion is accompanied by a decreased need for further glucose administration to maintain a satisfactory glycemic level during an insulin-induced hypoglycemic clamp [15]. Moreover, it improves triglyceride clearance and the sensitivity of adipose tissue to insulin, which may prevent ectopic fat deposition [16]. These facts paved the way for the development of a GIP receptor agonist-based therapy for T2DM, looking also for the possibility of finding a dual GLP-1/GIP receptor agonist [17].
Similar to GLP-1 R’s, there appears to be GIP receptors located in the brain and CNS. Thus, it’s possible that the feeling of satiety with the use of Tirzepatide may be dictated by binding to GIP-R’s in the brain.
A review from Fukuda, M.5 outlines some of the works which have elucidated GIP-R’s within the brain, such as in the excerpt below:
GIPR expression has been observed throughout the brain, including CNS sites responsible for energy metabolism. An early autoradiographic analysis of [125I]GIP identified putative GIPR binding sites in several brain regions (43) but failed to detect GIP binding in the hypothalamus. Other methodologies, such as in situ hybridization, Northern blot, and quantitative PCR (qPCR), also showed broad CNS distribution of GIPR mRNA and included the hypothalamus (13,44–47). Elegant work by Adriaenssens et al. (48) recently revealed the anatomical distribution of GIPR in the CNS at a cellular resolution. They generated a Cre-dependent reporter mouse that enabled the identification of Gipr-positive cells and observed Gipr expression throughout the CNS, including the hypothalamic nuclei (the ARC and dorsomedial and paraventricular nuclei of the hypothalamus) and hindbrain areas that are involved in energy balance. Because this model is a germ line reporter, transient expression of Cre recombinase during development may be possible. Nevertheless, these data suggest that GIPR is present in the CNS regions that are responsible for energy homeostasis and support a CNS role for GIPR in the control of energy balance.
It’s fascinating to consider how widespread these incretin receptors are, and provides some insights into a neuronal component to fullness.
Incretins as the next frontier for accelerated approval
It should come as no surprise that Tirzepatide is being met with widespread attention. These classes of drugs may actually be the miracle weight-loss drug that many people jokingly talk about in passive conversations.
However, caution should be warranted with such so-called “miracle” drugs.
There’s been a growing push to accelerate approval of various medications, with many of these medications being those which have the highest level of public interest.
Of note, two Alzheimer’s immunotherapies from Biogen/Eisai have received accelerated approval even as these two drugs have come under serious scrutiny as covered previously:
Mired in Controversy over Aducanumab, Biogen reports "positive" results over their new Alzheimer's Immunotherapy Lecanemab
Modern Discontent is a reader-supported publication. To receive new posts and support my work, consider becoming a free or paid subscriber. Edit 12/5/2022: Apparently one of the headers below had the …
Pharmaceutical manufacturers, as well as regulatory bodies such as the FDA, look to the public to see what cures people are yearning for. Whether Alzheimer’s, cancer, or weight-loss, it is the public that partially dictates what research gets funding as well as what drugs make it to the market, with many of these companies eager to satiate the wants of the masses irrespective of the possible harms or side effects.
As summer approaches there’s no doubt that Tirzepatide and similar drugs will receive more and more coverage, providing a quick fix to having that beach-ready body.
I should warn people that the SURMOUNT-2 trial carried out by Eli Lilly took place over the course of 72 weeks, so maybe you won’t be ready in time for this summer.
But that’s probably for the best. It’s become the norm to turn to drugs to aid an otherwise arduous process. Medications aren’t inherently bad, but may be overprescribed and abused in lieu of other avenues, especially ones that may sustain a more healthful lifestyle such as changes in diet or exercise.
It should also be reminded that these drugs are, by their very nature, hormones, and with a lack of long-term safety data one should be concerned about alterations in hormones such as the possibility of incurring GIP or GLP-1 resistance akin to insulin resistance formation in Type-II diabetics.
So as the summer days draw near and pharmaceutical manufacturers push for the Summer of 2023 to be an incretin summer, be mindful of whether you are being taken advantage of. There are other avenues to losing weight, and even if you choose to take these medications remember that the long-term effects, including maintenance of weight-loss, are unknown.
Sometimes it’s better to go it the long way, and sometimes that makes the experience all the more rewarding.
Note: I am not a doctor. Please consult with proper medial professionals when considering medications. The intent of this information is to be informative not prescriptive.
Substack is my main source of income and all support helps to support me in my daily life. If you enjoyed this post and other works please consider supporting me through a paid Substack subscription or through my Ko-fi. Any bit helps, and it encourages independent creators and journalists such as myself to provide work outside of the mainstream narrative.

For this review RA serves as a shorthand notation for Receptor Agonist. Remember that an agonist is a molecule which activates a receptor (in contrast to an antagonistic molecule). In places where R is used it will refer just to Receptors. The use of these shorthand notations may be done interchangeably within this review so keep that in mind if it causes confusion.
Chavda, V. P., Ajabiya, J., Teli, D., Bojarska, J., & Apostolopoulos, V. (2022). Tirzepatide, a New Era of Dual-Targeted Treatment for Diabetes and Obesity: A Mini-Review. Molecules (Basel, Switzerland), 27(13), 4315. https://doi.org/10.3390/molecules27134315
Forzano, I., Varzideh, F., Avvisato, R., Jankauskas, S. S., Mone, P., & Santulli, G. (2022). Tirzepatide: A Systematic Update. International journal of molecular sciences, 23(23), 14631. https://doi.org/10.3390/ijms232314631
Fisman, E. Z., & Tenenbaum, A. (2021). The dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist tirzepatide: a novel cardiometabolic therapeutic prospect. Cardiovascular diabetology, 20(1), 225. https://doi.org/10.1186/s12933-021-01412-5
Fukuda M. (2021). The Role of GIP Receptor in the CNS for the Pathogenesis of Obesity. Diabetes, 70(9), 1929–1937. https://doi.org/10.2337/dbi21-0001





For heaven’s sake, if you want to,lose weight stop eating crap. And if you need to control your blood sugar/glucose levels try berberine and milk thistle. Works better than metformin with fewer side effects.
I would give these a hard pass. There is a consequence to throwing anything out of balance.
They promote systemic upregulation of proinflammatory and oncogenic & autoimmune related interleukin 6 (il-6).
Il-1 is also oncogenic.
It's also one of the reasons why cancer patients waste away. Yeah.
***
Glucagon-like peptide 1 receptor induced suppression of food intake, and body weight is mediated by central IL-1 and IL-6
(2013)
...Glucagon-like peptide 1 (GLP-1), produced in the intestine and the brain, can stimulate insulin secretion from the pancreas and alleviate type 2 diabetes. The cytokine interleukin-6 (IL-6) may enhance insulin secretion from β-cells by stimulating peripheral GLP-1 production. GLP-1 and its analogs also reduce food intake and body weight, clinically beneficial actions that are likely exerted at the level of the CNS, but otherwise are poorly understood. The cytokines IL-6 and interleukin 1β (IL-1β) may exert an anti-obesity effect in the CNS during health. Here we found that central injection of a clinically used GLP-1 receptor agonist, exendin-4, potently increased the expression of IL-6 in the hypothalamus (11-fold) and the hindbrain (4-fold) and of IL-1β in the hypothalamus, without changing the expression of other inflammation-associated genes.
https://pubmed.ncbi.nlm.nih.gov/24048027/
***
Oncogenic Ras-induced secretion of IL6 is required for tumorigenesis
...IL6 appears to act in a paracrine fashion to promote angiogenesis and tumor growth. Inhibiting IL6 may therefore have therapeutic utility for treatment of cancers characterized by oncogenic Ras mutations.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1920165/
***
Roles of IL-1 in Cancer: From Tumor Progression to Resistance to Targeted Therapies
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7503335/
***
You know how you lose your appetite when you are ill? You really don't want to recreate these illness factors:
Inflammatory cytokines, appetite‑regulating hormones, and energy metabolism in patients with gastrointestinal cancer
https://www.spandidos-publications.com/10.3892/ol.2020.11662
***
Plasma concentration of interleukin-6 was upregulated in cancer cachexia patients and was positively correlated with plasma free fatty acid in female patients
https://nutritionandmetabolism.biomedcentral.com/articles/10.1186/s12986-019-0409-9
***
Weight loss via your bones is not good:
Interleukin-1 receptor antagonist decreases bone loss and bone resorption in ovariectomized rats.
...Interleukin-1 (IL-1), a cytokine produced by bone marrow cells and bone cells, has been implicated in the pathogenesis of postmenopausal osteoporosis because of its potent stimulatory effects on bone resorption in vitro and in vivo.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC294303/