“Exercise in a Pill”
A recent study may explain why Metformin may help people lose weight, and why some pause should be given to this mechanism of action.
This is a dive into a rather newly discovered class of compounds, in particular one called Lac-Phe, which have been found as a byproduct of intense exercise. Evidence has eventually found that Lac-Phe may have an influence on hunger and leading researchers to look deeper into what these molecules can do. Interestingly, a recent study has suggested that Lac-Phe may also be produced by the anti-diabetic drug Metformin. Given the popularity of weight-loss I wanted to look at what was going on with Lac-Phe and Metformin. I may examine Metformin to a bigger degree some time in the future given how popular of a drug it is. Consider this a dive into a new field of research.

An Exercise Molecule that Suppresses Appetite?
Given the discourse around GLP-1 RAs I have become a bit interested in other alleged weight-loss medications. I’ve been meaning to investigate Metformin because of it being an FDA-approved medication derived from nature, juxtaposing the talk around Berberine. Unfortunately, I never really had the time to do as deep of an investigation as I would like.
So, it could be considered good timing that an article was published recently in Nature Metabolism1 suggesting one way in which Metformin appears to aid in weight-loss and appears to be related to a recently discovered molecule related to exercise called Lactoylphenylalanine, or Lac-Phe for short.2
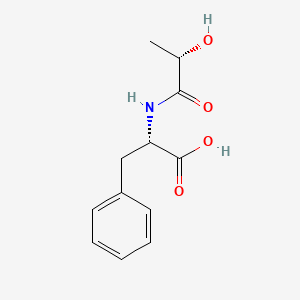
And short is a rather appropriate term here. Lac-Phe is a pseudo-dipeptide meaning that it is comprised of what would appear to be two constituents- lactic acid and phenylalanine. Phenylalanine itself is an amino acid, but lactic acid serves as a byproduct of alternative metabolic pathways such as lactic acid fermentation.
It’s here where Lac-Phe finds its association to exercise. During strenuous exercise several factors are at play- the body may not be able to transport oxygen quick enough to muscles so energy is made by way of anaerobic respiration, the metabolism of glycolysis may lead to excess buildup of pyruvate, and cofactors such as NADH may have to be recycled to be reused for glycolysis.
In any case, strenuous exercise leads to the formation of lactate by way of an alternative metabolic pathway, and it’s the lactate that forms which is related to the soreness you experience while working out.
Supposedly, as llactate begins to buildup it may encounter an enzyme called Cytosolic non-specific dipeptidase (aka carnosine dipeptidase 2/ CNDP2). Normally CNDP2 serves as a manganese-dependent metallopeptidase that breaks down dipeptides into amino acid constituents. However, it has been found that CNDP2 is also responsible for generating N-lactoyl amino acids in a process known as reverse proteolysis.
The discovery of this process process itself is also rather recent, as the pivotal study which discovered these N-lactoyl amino acids was published in 2015.3 This appears to be due to the fact that most studies that have discovered these N-lactoyl amino acids miscategorized these molecules as being derived from 1-carboxyethyl-amino acids rather than being derived from lactate.
Although researchers discovered Lac-Phe they were still unsure of what biochemical mechanisms this molecule serves. It wasn’t until a very recently published study in 2022 did researchers tie Lac-Phe to weight-loss.4
This pivotal study was comprised of some of the same members from the recent Metformin study. The investigation into Lac-Phe was based on various bits of evidence, such as the fact that there appears to be a correlation between BMI and two missense polymorphisms in CNDP2, suggesting that CNDP2 may play a role in energy homeostasis which may be perturbed in those carrying these mutations, thus tying CNDP2 with one of its metabolites Lac-Phe as possibly being related to obesity in some manner.
The study is rather long, but in short researchers examined whether exercise in monkeys, horses and humans would induce Lac-Phe production.
For humans two cohorts were used- one in which people were tasked with running on a treadmill and another in which people were tasked with endurance, sprint, and resistance exercises. Both cohorts compared Lac-Phe levels to those who did not exercise and noted an elevated level of Lac-Phe up to an hour post-strenuous exercise. Of note, the effects here appear to be related predominately to intense cardio exercises as resistance and endurance training did not appear to produce as much Lac-Phe. This is a rather interesting finding given that strength training is known to accumulate a large degree of lactate (again, the muscle soreness that comes from fatigue while performing a set of some sort of workout), so it's curious if not all lactate production are equal, but this may be related to the intensity of the exercise.

This at least confirms that exercise is related to the production of Lac-Phe. However, it’s worth noting that serum concentrations of both Lactate (the precursor) and Lac-Phe are not directly one-to-one. That is, Lac-Phe levels appeared higher than Lactate levels by the 15-minute post-exercise timepoint for the treadmill runners and continues to remain elevated while lactate levels decline. This may be due to removal of lactate and a possible long half-life of Lac-Phe. It could also be related to intracellelular/extracellular levels of Lactate vs Lac-Phe.
The authors further noted that several cell types including macrophages, epithelial cells, and CNDP2+ cells may be involved in biosynthesis of Lac-Phe. It’s possible that Lactate released from muscles can then be taken up by these cell types and converted into Lac-Phe.
Now, what’s interesting is that when Lac-Phe was administered to obese mice researchers noticed a decline in food consumption, and this decline continued through continual administration of Lac-Phe. Interestingly, this effect didn’t appear to occur in lean, chow-fed mice. Water consumption also did not appear to decline in the obese mice, suggesting that nausea may not have been a reason for the lack of eating.

Although rather impressive, the dosage of Lac-Phe used in the study equated to inducing circulating Lac-Phe levels of 50 mg/kg body weight- far above what would be achieved from exercise alone. Thus, if a dose-dependent response is needed to induce appetite suppression there’s a question whether this appetite-suppressing effect of Lac-Phe is achievable with exercise alone. However, the relatively low levels of Lac-Phe may be balanced by the fact that several N-lactoyl-peptides have been discovered, all of which may contribute to the overall appetite suppressing effect if they so happen to operate in a similar manner as Lac-Phe. It’s only Lac-Phe that appears to be the focus of much of the research that is going on.
There’s also the question of where Lac-Phe may be acting. A commentary article from Lund, et al.5 posits that these N-lactoyl peptides may bind to G protein-coupled receptors on neurons and therefore may be involved with sensory regulation of appetite:
How does Lac-Phe affect appetite? Where is it produced during exercise? And what is its primary target cell and receptor? CNDP2 is expressed in many different tissues of the body (Jansen et al., 2015), but Li et al., 2022 identify immune and epithelial cells as the potential main sites of Lac-Phe biosynthesis through single-cell transcriptomic database analysis. Regarding the molecular mechanism, a likely target for Lac-Phe is a G protein-coupled receptor (GPCR) sensor expressed on CNS neurons involved in appetite regulation (Figure 1B). Several GPCRs that recognize carboxylated metabolites or oligopeptides could, in principle, function as sensors for Lac-Phe and potentially other L-lactate-derived pseudo-dipeptides (Husted et al., 2017), but that remains to be experimentally addressed. Identification of a receptor or other molecular targets for Lac-Phe would allow for more specific genetic and pharmacological evaluation of both physiological and pharmacological effects of Lac-Phe.
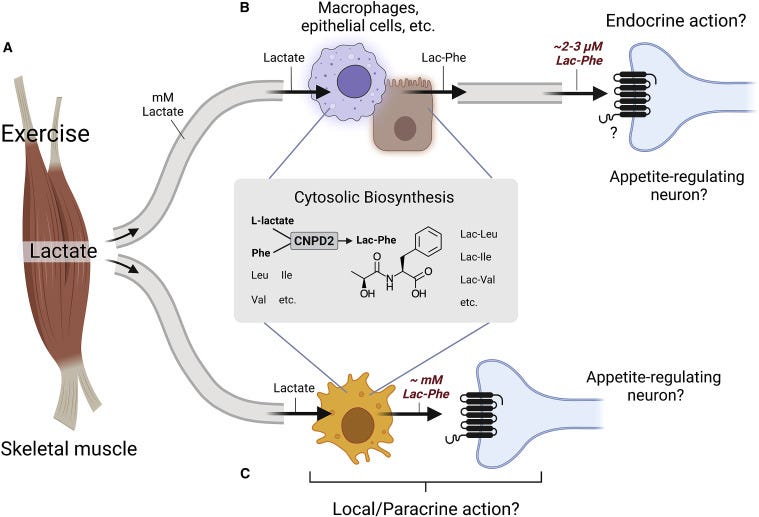
The commentary provides more regarding what role Lac-Phe may play so check it out for further details.
This pathway is rather interesting since it overlaps in some ways to what is assumed to happen regarding GLP-1. It at least suggests that multiple agents may operate to stimulate neurons and produce an appetite-suppressing effect, although this process is still rather complex and not fully elucidated.
Overall, this study at least suggests a framework in which a byproduct of exercise can lead to hunger suppression and may help manage weight.
Of course, one may ask why such a mechanism may exist?
As in, why would there be a metabolite from exercise that would make someone feel less hungry? Shouldn’t the energy expenditure lead to an increase in appetite?
There’s plenty regarding these N-lactoyl peptides that are yet to be elucidated, but on the surface I’m curious if the response is likely associated with sympathetic nerve activation. That is, from an evolutionary standpoint it’s likely that strenuous exercise and sprinting may occur to escape predators or chase after prey, and in that regard directing energy towards the stomach and away from muscles may not be beneficial. Essentially, Lac-Phe may be a byproduct of the fight or flight response that may have
Metformin and Lac-Phe
This brings us to the currently released study. Again, we will be referring to the preprint in the meantime.6
The study relied on blood samples taken from previous cohorts in which participants were provided Metformin, had blood drawn, and stored for use for other means. One cohort, the Stanford Cohort, examined participants from 1998-2004 who had blood samples taken prior to taking metformin and 12 weeks post-metformin treatment. This was a small cohort in which the participants were described as Type-II diabetics, with the mean age being around 58 with a rather surprisingly low mean BMI of 29.4.
Metformin was administered twice a day at a dose of 500 mg and was increased to 2g/day.
As would be expected, administration of Metformin led to an increase of circulating Metformin among the Stanford Cohort. Coinciding with this increase in circulating Metformin participants also had increased levels of Lac-Phe at the 12-week post-metformin administration mark, although the results here are sporadic.
Note that some participants showed a low increase in Lac-Phe, some people showed a high spike, and one participant showed relatively elevated Lac-Phe both pre/post metformin administration, being the highest peak relative to the other participants. Note that some participants, strangely, showed reduced levels of Lac-Phe at the 12-week mark which would contradict the initial hypothesis. Bear this in mind when considering the mean value of 0.32 ± 0.11 μM of Lac-Phen, which may be biased by some of the more extreme values.

It's important to note that the blood samples examined were also nearly 20 years old. This may not affect the results too much, but it’s curious why newer samples were not considered (in this case it may have been easier to find a cohort that already followed a pre/post-Metformin study method).
Another cohort was also examined including several thousand participants enrolled in a cohort study titled Multi-Ethnic Study of Atherosclerosis (MESA) which is looking at people from different ethnic backgrounds for subclinical characteristics of cardiovascular disease and examining risk factors that may lead to clinical evidence of heart disease.
Out of the nearly 6,000 participants enrolled into MESA around 179 were on Metformin. This at least tells us that this cohort may be on various medications other than Metformin. Nonetheless, when blood samples from those on Metformin were examined, there were higher levels of Lac-Phen relative to those who were not on Metformin. This also appears to be correlated with circulating Metformin levels as well- when Metformin participants were separated into quartiles based on circulating Metformin levels there appeared to be an increase in Lac-Phen levels.

Strangely, no demographic data is provided for the MESA Cohort including BMI, age, and even Metformin dose and duration of use. The units used also don’t appear to be in uM but appear to be based on regression models, so they aren’t directly comparable to the Stanford Cohort- we can only tell that Lac-Phen levels were higher among Metformin users relative to those who weren’t prescribed Metformin.
This is a problem in some regard because the researchers are looking at two separate cohorts but aren’t using the same measurements. They both intend to suggest that Lac-Phen levels are higher post Metformin come to such a conclusion using different methods.
Lastly, researchers note that Metformin did not influence circulating lactate levels. This is shown in Figure 1F which appears to be improperly labeled. It’s worth considering that the half-life of lactate may be far lower than Lac-Phe, and collection of blood must be considered with respect to Metformin use.
So as not to get bogged down in the rest of the study one key experiment investigated how Metformin induces lactate production. In this case, the authors point to Metformin’s alleged mechanism of action as being a mitochondrial complex I inhibitor.
Complex I is the first complex that makes up the electron transport chain of the mitochondria. It’s here where NADH from the Krebs cycle gets stripped of electrons and used to eventually reduce oxygen into water.

The connection between this complex and lactate production is still unclear- one may suggest that inhibition of Complex I may influence lactate production to free up NADH, or it may be a way of producing energy in lieu of Complex I inhibition. Nonetheless, the authors suspected that metformin’s inhibition of Complex I would lead to glycolytic flux in which cells would be directed away from cellular respiration and towards lactate production.
When Caco-2 and primary macrophages were provided Metformin in vitro there appeared to be shift away from cellular respiration at higher doses, which appeared to correlate with increased Lac-Phe levels.
The following graph is a bit confusing, but I’ll break it down a bit. Note that OCR refers to oxygen consumption rate and is a measure of how much oxygen is being used up during a set time. Hypothetically speaking, if Complex I is inhibited then glycolytic flux would lead to less consumption of oxygen as movement of electrons along the ETC would be hindered (note that oxygen Is needed in Complex IV).
When we look at Figure 3A note that at higher doses of Metformin OCR is reduced which would coincide with Complex I inhibition, with 1 mM- 10 mM Metformin levels showing comparable but heavily reduced OCR.

Note that researchers also utilized 3 different compounds which also affect the ETC. Oligomycin acts on ATP Synthase- the final step of the ETC in which protons that have been pushed into the intermembrane space of mitochondrial are pushed across a proton gradient to produce ATP for energy. Use of Oligomycin also pushes towards lactate production, and therefore likely acts synergistically with Metformin which explains why OCR was reduced with the use of Oligomycin. Rotenone is also a Complex I inhibitor and thus makes sense that administration of Rotenone too would lead to reduced OCR.
The interesting drug here is DNP, or 2,4-dinitrophenol. DNP became rather popular during the early 20th century when it was found that people working in DNP factories lost weight, leading to the use of DNP as a popular weight-loss drug. However, serious adverse effects were found with the drug and eventually they were banned in 1938. Today DNP may be obtained illicitly and is used by bodybuilders to help lose weight.
DNP’s mechanism of action is rather interesting: it serves as a mitochondrial uncoupler by shuttling protons across the mitochondria’s bilayer and thus bypassing ATP Synthase. This appears to be because DNP can pick up protons from the intermembrane space, move across the lipid bilayer, and remove the proton.7 This process is repeatable, and thus over time the proton gradient produced by the ETC is eventually lost.8

This uncoupling means that ATP cannot be produced and therefore signals a loss of possible energy for cells. Interestingly, it appears that this loss in energy causes the mitochondria to ramp up metabolism to compensate for the energy loss and thus explains some of the effects seen with DNP, including the production of excess heat by DNP users. Hyperthermia appears to be a rather common side effect of excess DNP use.
Essentially, the use of DNP in this experiment should ramp up metabolism and thus increase OCR. The pairing of Metformin with DNP emphasizes that oxygen consumption can be overcome, but at relatively high doses of Metformin even the use of DNP may not attenuate the glycolytic flux.
All this isn’t necessary for the study- it serves more to help get a better understanding for the study methodology. The main takeaway here is to confirm that Metformin inhibits Complex I, and it also appears that other inhibitors of the ETC may also contribute to Lac-Phe production as well, suggesting that Lac-Phe production may occur with other medications as well.
In addition, researchers noted an increase in Lac-Phe which appears to coincide with basal respiration inhibition as suggested in Figure 3B & C.
Refer to the preprint for more information regarding this part of the study, but what’s interesting is that researchers noted that, unlike exercise, Metformin appears to lead to intracellular production of Lac-Phe.
This contrasts exercise in which it is assumed that Lactate produced by myocytes gets released extracellular, picked up by cells such as macrophages, and then converted into Lac-Phe and released extracellularly and allowed to elicit its anti-hunger effects.

Further experimenting noted that knockout mice for CNDP2 were not affected by Metformin as food intake and body weight remained relatively consistent relative to wildtype mice which showed reduced food intake and loss of weight.
Overall, this study appears to suggest that weight-loss associated with Metformin may be related to production of Lac-Phe.
Precaution on Lac-Phe and Metformin
Over the past year or so Metformin has been made out to be a sort of wonder drug, and current research has investigated whether Metformin can help with cancer and even increase longevity. With comments suggesting that Metformin may help people lose weight there’s no doubt that Metformin may see even more interest, especially as drugs such as GLP-1 RAs have opened the door to miracle weight-loss drugs. Even though weight-loss with metformin appears modest relative to GLP-1 RAs the cost and relatively safe profile may make Metformin another popular drug for weight-loss, and maybe even more than Ozempic.
That being said, what’s concerning is that enthusiasm regarding this anti-hunger effect from Metformin comes without an understanding of why the body would produce Lac-Phe, and what reason would there need to be in order to produce this anti-hunger effect.
Again, why exactly would a molecule produced during strenuous exercise inhibit hunger? It seems paradoxical, and hence why I question whether this mechanism may be an evolutionary artifact of the fight or flight response. Thus, if Lac-Phe is tied to sympathetic activation then there may be a question regarding whether constant Lac-Phe release may pose some issue.
There’s much to consider given that Lac-Phe is a recently discovered molecule. Further research may help to better find answers for Lac-Phe’s mechanisms and whether these effects can extend to those that are not diabetic, and at that time we may better examine possible consequences of Lac-Phe. It’s important to consider the why and not just the what.
But as of now some hesitation should be considered regarding the reason for this anti-hunger mechanism existing. Precaution should be warranted before assuming that one should take metformin for weight loss before more evidence comes out regarding its MOA.
If you enjoyed this post and other works please consider supporting me through a paid Substack subscription or through my Ko-fi. Any bit helps, and it encourages independent creators and journalists such as myself to provide work outside of the mainstream narrative.

Xiao, S., Li, V.L., Lyu, X. et al. Lac-Phe mediates the effects of metformin on food intake and body weight. Nat Metab (2024). https://doi.org/10.1038/s42255-024-00999-9
The molecule is also named N-lactoyl-phenylalaline and refers to the fact that lactic acid is bonded to the amine from phenylalanine.
Jansen, R. S., Addie, R., Merkx, R., Fish, A., Mahakena, S., Bleijerveld, O. B., Altelaar, M., IJlst, L., Wanders, R. J., Borst, P., & van de Wetering, K. (2015). N-lactoyl-amino acids are ubiquitous metabolites that originate from CNDP2-mediated reverse proteolysis of lactate and amino acids. Proceedings of the National Academy of Sciences of the United States of America, 112(21), 6601–6606. https://doi.org/10.1073/pnas.1424638112
Li, V. L., He, Y., Contrepois, K., Liu, H., Kim, J. T., Wiggenhorn, A. L., Tanzo, J. T., Tung, A. S., Lyu, X., Zushin, P. H., Jansen, R. S., Michael, B., Loh, K. Y., Yang, A. C., Carl, C. S., Voldstedlund, C. T., Wei, W., Terrell, S. M., Moeller, B. C., Arthur, R. M., … Long, J. Z. (2022). An exercise-inducible metabolite that suppresses feeding and obesity. Nature, 606(7915), 785–790. https://doi.org/10.1038/s41586-022-04828-5
Lund, J., Clemmensen, C., & Schwartz, T. W. (2022). Outrunning obesity with Lac-Phe?. Cell metabolism, 34(8), 1085–1087. https://doi.org/10.1016/j.cmet.2022.07.007
Lac-Phe mediates the anti-obesity effect of metformin
Shuke Xiao, Veronica L. Li, Xuchao Lyu, Xudong Chen, Wei Wei, Fahim Abbasi, Joshua W. Knowles, Shuliang Deng, Gaurav Tiwari, Xu Shi, Shuning Zheng, Laurie Farrell, Zsu-Zsu Chen, Kent D. Taylor, Xiuqing Guo, Mark O. Goodarzi, Alexis C. Wood, Yii-Der Ida Chen, Leslie A. Lange, Stephen S. Rich, Jerome I. Rotter, Clary B. Clish, Usman A. Tahir, Robert E. Gerszten, Mark D. Benson, Jonathan Z. Long
bioRxiv 2023.11.02.565321; doi: https://doi.org/10.1101/2023.11.02.565321
Molecules that exhibit this mechanism of action are called protonophores.
Borisov, Evgeny & Bezsonov, Evgeny & Lyukmanov, Damir & Poggio, Paolo & Moschetta, Donato & Valerio, Vincenza. (2022). Pharmacological agents affecting mitophagy and inflammation. Vessel Plus. 6. 10.20517/2574-1209.2022.20.



Thank you for looking into this! It is looking less and less ethical all the time...kind of like that genetic manipulation injection some people have been tricked into taking...