Evidence of myocarditis and pericarditis with Novavax Antigen-based Vaccine
And the importance of comparing adverse reactions across various vaccine platforms for similar adverse mechanisms.
Since the rollout of the spike-based Novavax vaccine reports of adverse reactions have flown under the radar. Here in the US most reports have focused on the mRNA-based vaccines as evidence continues to provide a concerning safety signal.
Novavax, unlike the mRNA vaccines, are more in line with traditional vaccines as they rely on Wuhan spike studded within a lipid nanoparticle, thus the antigen/spike-based vaccine moniker. Although the lipid nanoparticle technology is synonymous with the mRNA vaccines, Novavax is predominately composed of cholesterol, phosphatidylcholine, and a plant-derived saponin called Quillaja saponin, used in many foods and cosmetics.
More information on Novavax can be found in a prior post:
Given this mechanism in which Novavax does not rely on host cells for production there’s been an assumption that Novavax would, hypothetically, report with a much better safety signal.
However, soon after the rollout employees at the FDA raised concerns about possible heart issues after receiving the Novavax vaccine, and more importantly anecdotal reports of cardiovascular issues post-Novavax vaccination popping up in Australia would corroborate this concern, as Australia saw a greater use of Novavax relative to other countries.
Now, recent evidence appears to suggest a possible association between the Novavax vaccine and instances of myocarditis and pericarditis. Again, the information is limited, but the fact that such information aligns with mRNA vaccines suggests a similar mechanism of COVID mimicry across all platforms.
Myocarditis/Pericarditis Evidence
The evidence emerging so far with respect to Novavax is very limited, with a few case reports and one retrospective analysis available aside from anecdotes.
Case report of 30 year-old male
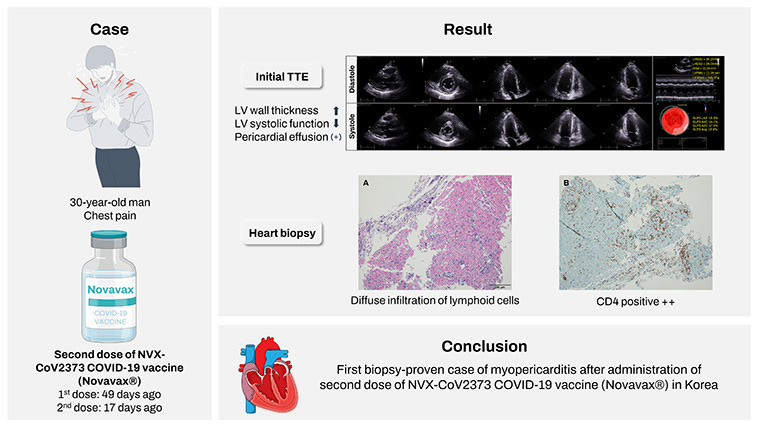
One case report of myopericarditis1 comes from a 30-year-old Korean man who began experiencing severe chest pain 17 days after receiving a second dose of the Novavax vaccine:
A 30-year-old man was referred to our emergency department with complaints of chest pain and mild febrile sense for two days. The patient received the first dose of NVX-CoV2373 COVID-19 vaccine (Novavax®) 49 days ago and the second dose 17 days ago. The characteristic of the pain was sharp and as intense as NRS (Numeral Rating Scale) 7, and it was located in the substernal region of the central anterior chest. The pain was worsened by dry cough or deep breathing, and was relieved by rest. The patient had medical history of ulcerative colitis and was taking 1 gram of mesalazine twice a day for five years. The patient denied any recent bowel habit changes including stool frequency and rectal bleeding or use of new medications.
Serum troponin-I levels were elevated along with creatine kinase-MB type, which are markers typically associated with heart damage.
Further assessment via echocardiography showed left ventricular thickness as well as pericardial effusion (unusual fluid in the pericardial cavity of the heart).
This led researchers to conduct an endomyocardial biopsy which showed lymphoid infiltration and the presence of CD4+ cells (seen in the stains above).
The exact mechanism of this myopericarditis is not fully understood, and may either be due to the COVID mimicry of the spike or through an immunological response.
What’s interesting is the biopsy which noted the T-cell infiltration, suggesting that an immune-mediated targeting of the heart may have been possible.
In this case the patient received corticosteroids daily for the first 5 days of hospitalization followed by weekly administration. Afterwards, troponin levels and LV thickness returned to normal and the patient was released from the hospital 12 days after admission.
Inflammation from Novavax after BioNTech Inflammation
In a strange case series from Ahmad et al.2 two people who had experienced myopericarditis (both myocarditis and pericarditis) following vaccination with a second dose of BioNTech’s mRNA vaccine were given a Novavax booster and experienced myopercarditis as well following this booster.
One case was of a 26-year old man who presented with chest pains and shortness of breath 11 days after his second BioNTech vaccine. The symptoms appeared 5 days after the second dose and worsened, eventually persisting for months. The man was diagnosed with a case of pericarditis.
Six months after this event he received a Novavax booster and began experiencing symptoms once again:
Six months after this episode of pericarditis, he received his booster vaccination with NVX-CoV2373. Prior to vaccination he was symptom free. He reports onset of similar symptoms, to the prior episode of pericarditis, with pleuritic chest pain and dyspnoea 2–3 days post NVX-CoV2373 vaccination. Examination and laboratory cardiac biomarkers were once again normal (Troponin T 4 ng/L, NT-Pro-BNP < 50 ng/L), however CRP was elevated at 33.2. ECG demonstrated global ST elevation and bedside echocardiogram was normal. He was diagnosed with pericarditis in the Emergency Department (Brighton Criteria for Pericarditis level of certainty 2) [8] and was treated with non-steroidal anti-inflammatories and recommenced on colchicine. He had persistent symptoms for approximately 2 months. Interestingly, he contracted COVID-19 infection 2 months after this second episode of pericarditis, with symptoms of a mild upper respiratory tract infection but no recurrence of the symptoms of pericarditis.
In one 25-year old woman symptoms of adverse reactions began 48 hours after she received a second BioNTech dose, which was diagnosed as myopericarditis due to troponin I levels, abnormal cardiac MRI findings, and presence of myopericardial oedema (fluid build-up).
She was placed on steroids and continued to present with symptoms for five months, which included several hospital visits over this time period.
She, too, would eventually receive a Novavax booster and presented with symptoms once again days afterward (emphasis mine):
Repeat MRI 9 months later was normal with complete resolution of the previously seen abnormalities (Fig. 2), as well as normalisation of Troponin I levels. She also remained symptom free and subsequently undertook booster vaccination with NVX-CoV2373. Five days post vaccination, she once again developed progressive severe symptoms of intermittent palpitations, dyspnoea, and chest pain. This was associated with acute elevation in cardiac biomarkers (Troponin I 349 ng/L and NT-ProBNP 347 ng/L) and a diagnosis of myocarditis was made (Brighton Criteria level 2) [8]. Echocardiogram was normal with preserved ventricular size and function. She was recommenced on tapering prednisolone regimen with subsequent gradual improvement of her symptoms. Rapid taper of prednisolone was associated with flare of symptoms and repeat hospital presentations. She reports ongoing exertional symptoms that have persisted to date at 2 months post diagnosis and vaccination.
The presentation of symptoms here is rather interesting. It follows two doses of an mRNA vaccine with a follow-up booster of a non-mRNA vaccine due to adverse reactions, which appear to be the recommendation made by Australian medical authorities (emphasis mine):
Importantly, both of our subjects had clinical resolution of symptoms from their reaction following BNT162b2 for at least 3 months, with subject 2 also demonstrating radiological resolution, before again having myopericarditis after receiving NVX-CoV2373. This signifies that both subjects fit current Australian recommendations for receiving a subsequent non-mRNA vaccine following post mRNA vaccine myopericarditis.
However, the similarities in symptoms raise serious questions as to what the actual mechanisms for these adverse reactions are.
There’s one consideration towards COVID mimicry induced by the spike antigen, but one has to remember that the spike should, hypothetically, be bound by anti-spike antibodies. Therefore, although the spike may be present post-vaccination current anti-spike antibodies should prevent spike pathogenicity (again, hypothetically).
This may be partially explained by the presence of symptoms after the second dose and after each repeat dose. However, since the immunological profile of these two cases were not reported there’s no available evidence towards unbound spike effect.
Rather, it’s possible that the adverse reactions are possibly immune-mediated with adjuvants inducing a greater immunological effect that may be damaging to those who are more susceptible.
Although Ahmad et al. notes that both Novavax and BioNTech use adjuvant-based lipid nanoparticles the adjuvants are different (again, Novavax uses a plant-based saponin while BioNTech uses a ionizable amine), and therefore either a rather broad adjuvant effect is occurring, or the effect may be independent of adjuvants and may be related more to a hyper immune response in individuals.
These remarks are summarized, in part, below by Ahmad et al.:
One theory that encompasses both myopericarditis following a COVID-19 infection, as well as post-COVID-19 vaccination, is molecular mimicry between the spike protein and the self-antigen, alpha-myosin, causing direct injury to the myocardium. As this hypothesis does not account for the observed skew towards mRNA vaccines, an alternative theory proposes that the immunogenicity of the mRNA strands as the cause for immune dysregulation and subsequent indirect damage to the myocardial tissue, in predisposed individuals [9].
More recently, another theory has flagged the immunogenicity of the lipid nano particle (LNP) sheath required to deliver the mRNA molecule to host cells in mRNA vaccines, as a potential cause for either direct damage to the myocardial cells, or as another trigger for immune dysregulation. Interestingly, the LNP sheath is also used in the NVX-CoV2373 vaccine, which is required for incorporation of the S-protein into the host [10].
As a shared pathomechanism would tend to lead to similar clinical presentations, it is interesting that both our subjects had mirroring events following their BNT162b2 and NVX-CoV2373 vaccinations. As young individuals who had reactions within 7 days of a non-first dose vaccination, both also fit the same demographic usually seen in post-mRNA vaccine myopericarditis. Both fulfill the criteria of myopericarditis, with presentations of pleuritic chest pain and ECG changes, although subject 1 had a mildly raised CRP and normal troponins, while subject 2 had a normal CRP with markedly high troponins.
Retrospective Analysis
Last week a retrospective analysis was published3 looking at adverse reactions reported in the WHO’s global database of individual case safety reports (ICSRs). The focus of this analysis was to compare Novavax myocarditis/pericarditis rates with those of the other vaccines.
The analysis searched for adverse reports associated with the mRNA, adenoviral, and antigen-based vaccines by searching for instances of myocarditis, pericarditis, and myopericarditis. The mRNA vaccines served as a positive control due to their association with increased risk of heart inflammation.
From the search over 61 myopericarditis ISCRs were associated with the Novavax vaccine, with most reports coming from Australia and some due to heterologous vaccination:
Following our search, 61,812 ICSRs of myopericarditis were found. From these, 61 ICSRs included the NVX-CoV2373 vaccine; 45 (73.8%) reported pericarditis, 11 (18.0%) myocarditis, four (6.6%) myopericarditis and one (1.6%) both terms (myocarditis and pericarditis) (Table (Table1).1). For nine ICSRs (14.8%), there was more than one suspected COVID-19 vaccine (i.e., heterologous vaccination regimens including NVX-CoV2373 and other suspected COVID-19 vaccines): five (55.6%) indicated mRNA-1273 vaccine (Moderna) and four (44.4%) BNT162b2 vaccine (Pfizer-BioNTech). In four cases, NVX-CoV2373 was utilized as a booster: two were administered after mRNA-1273 and two after BNT162b2. Only three ICSRs (4.9%) mentioned concomitant medication (other than vaccines): tranexamic acid, cannabidiol and flecainide.
Most of the reports were either of unknown origin or from consumers/non-health professionals.
When comparing reporting odds ratios the researchers noted a disproportionate increase for Novavax vaccines similar to mRNA vaccines. Note that “similar” here doesn’t mean numerically similar, but that both vaccines appear to show a disproportionate increase when compared to something such as the adenoviral vaccines:
Similar to VAERS, remember that the odds ratios calculated are heavily contingent upon reporting of the adverse reactions.
Different Vaccines; Similar Adverse Reactions
So far, the limited evidence published points towards concerning safety signals with respect to the antigen-based Novavax vaccines.
The higher reports of myocarditis and pericarditis associated with the mRNA vaccines in global databases of adverse reactions may be due to sampling bias, given that most people were provided these vaccines compared to the adenoviral and antigen-based vaccines.
Nonetheless, the fact that several different vaccine platforms have shown evidence of heart inflammation suggests a possible shared mechanism between them all.
Again, one of the main hypotheses may suggest direct pathogenic mechanisms regarding the spike protein via the spike effect. Given that the adenoviral and mRNA-based vaccines require host cellular machinery to produce the spike one would inherently assume that more spike may be presented from these vaccines relative to Novavax.
That’s more of a speculation, but the evidence suggests that myocarditis and pericarditis rates among adenoviral vaccine recipients aren’t disproportionately increased, which raises questions as to why these vaccines in particular don’t show the same rates of adverse reactions.
This would also have to be contextualized with an anti-spike immunological response that would hypothetically bind the spike and prevent it from binding to host cells and receptors. Therefore, in contrast to the spike effect a sponging effect should be considered. It’s possible that repeat exposure through vaccination may provide additional opportunities for escape which aren’t provided by the one-dose adenoviral vaccines, but again to what extent a sponging effect should eliminate free-roaming spike should be considered.
To that, one other hypothesis has been made which infers that LNPs containing spike mRNA may invade other tissues such as the heart, induce production of spike in this region, and then an anti-spike response from the host immune system that targets transfected cells leading to heart inflammation.
This could be a possibility given that there’s a chance that the LNPs may not remain at the site of injection. However, the Novavax biopsy from Kim et al. notes lymphoid and CD4 infiltrates for a Novavax recipient, which cannot be rationalized by transfection with Novavax given that it’s antigen-based and therefore should not be able to manipulate host cell machinery.
Given these issues with these hypotheses, one viable hypothesis may point towards some sort of immunological response. Questions surrounding this hypothesis includes autoimmunity or a hypersensitive response that may cause an overreaction of the immune system which damages tissues and organs.
To many of these hypotheses, many questions are still being left unanswered as to what could actually be occurring. It’s likely a mix of various different mechanisms, wholly dependent upon the individual which may dictate the adverse reaction.
However, given some of the recent information I’ve come across, including the result from the Kim et al. biopsy, I’m going to entertain the idea of an immunological basis of adverse reactions.
Apologies for those wanting a series on viral parkinsonism! I’ll blame it on ADHD or something related, but in finding some of the information I have I think it’s probably worth developing a hypothesis towards the adverse reactions.
Substack is my main source of income and all support helps to support me in my daily life. If you enjoyed this post and other works please consider supporting me through a paid Substack subscription or through my Ko-fi. Any bit helps, and it encourages independent creators and journalists such as myself to provide work outside of the mainstream narrative.

Kim, H. Y., Cho, J. Y., Yoon, H. J., Choi, Y. D., Ahn, Y., Jeong, M. H., Cho, J. G., & Kim, K. H. (2022). A Case Report for Acute Myopericarditis After NVX-CoV2373 (Novavax®) COVID-19 Vaccination. Journal of Korean medical science, 37(34), e265. https://doi.org/10.3346/jkms.2022.37.e265
Ahmad, S., Yuson, C., Le, A., & Hissaria, P. (2022). Myopericarditis following both BNT162b2 and NVX-CoV2373. Allergy, asthma, and clinical immunology : official journal of the Canadian Society of Allergy and Clinical Immunology, 18(1), 109. https://doi.org/10.1186/s13223-022-00750-7
Macías Saint-Gerons, D., Ibarz, M. T., Castro, J. L., Forés-Martos, J., & Tabarés-Seisdedos, R. (2023). Myopericarditis Associated with the Novavax COVID-19 Vaccine (NVX-CoV2373): A Retrospective Analysis of Individual Case Safety Reports from VigiBase. Drugs - real world outcomes, 1–8. Advance online publication. https://doi.org/10.1007/s40801-023-00355-5




Wow. It's almost like these shots should have been tested for all these possibilities before being given to billions of people.
My cat suffered from acute heart failure at age 6 months and was diagnosed with ‘transient myocardial thickening’ and had a raised troponin level, which I was told could be provoked by vaccination ( occurred 4 & 3 months prior) or general anaesthetic ( occurred 2 months prior). I’m inclined to blame his vaccines as he also developed a weird skin rash on his face after the first vaccines at age 2 months ( specialist dermatologist at vet hospital couldn’t diagnose it). He clearly didn’t have a covid vaccine but still probably had a myocarditis type episode. Given that the smallpox/monkeypox vaccines have a higher rate of myocarditis too I think there is clearly a generic risk to the heart from vaccination. Maybe some of it can be explained by Marc Girardot and his ‘bolus theory’.