COVID Vaccines and Parkinson's Disease Part II
An overview of Parkinson's Disease and Parkinsonism.
This is Part II of the ongoing series contextualizing COVID-19, COVID-19 vaccines, and Parkinson’s Disease. Part I can be found here.
Here we’ll look at an overview of Parkinson’s disease and a broader syndrome known as parkinsonism. The information provided cannot be all-encompassing so additional resources will be included for those interested. Note that Parkinson’s disease, Parkinson’s, and PD will all be used interchangeably within this series so recognize that they all refer to the same concept.
Parkinson’s Disease Overview
Signs/Symptoms and Diagnosis
Parkinson’s disease (PD) is the second most common neurodegenerative disease known second to Alzheimer’s dementia.
PD is generally recognized for the motor movement issues related to the disease, usually manifesting as resting tremors (tremors in the hands and feet that occur when one is still but disappears when one moves), stiffness in the body, or slowness of movement known as bradykinesia.
Its name is derived from its discoverer James Parkinson who first described the disease in an 1817 essay titled An Essay on the Shaking Palsy1:
So slight and nearly imperceptible are the first inroads of this malady, and so extremely slow its progress, that it rarely happens, that the patient can form any recollection of the precise period of its commencement. The first symptoms perceived are, a slight sense of weakness, with a proneness to trembling in some particular part; sometimes in the head, but most commonly in one of the hands and arms. These symptoms gradually increase in the part first affected; and at an uncertain period, but seldom in less than twelvemonths or more, the morbid influence is felt in some other part. Thus assuming one of the hands and arms to be first attacked, the other, at this period becomes similarly affected. After a few more months the patient is found to be less strict than usual in preserving an upright posture: this being most observable whilst walking, but sometimes whilst sitting or standing. Sometime after the appearance of this symptom, and during its slow increase, one of the legs is discovered slightly to tremble, and is also found to suffer fatigue sooner than the leg of the other side: and in a few months this limb becomes agitated by similar tremblings, and suffers a similar loss of power.
This essay would eventually lead to further research and recognition of PD. As of now, it is suspected that over 1 million Americans are living with PD with an range of 1-2% of those over the age of 50 being afflicted with the disease. Of course, one of the most well-recognized actors to have it being Michael J. Fox who started the Michael J. Fox Foundation for Parkinson’s Research:
Similar to Alzheimer’s PD is considered to be an age-related disease generally striking those starting in their 50s, making this a disease of increasing concern as many countries deal with an aging population. However, PD can be diagnosed at younger ages, with those diagnosed in the age range of 20s-50s being recognized as early-onset PD. Younger ages would be considered juvenile-onset PD.
PD is complicated to diagnose and generally relies on the presentation of motor movement issues, and may rely on recognition by friends and family that something is out of the ordinary by looking for the above signs, as well as other factors such as a loved one tripping and falling more often than normal.
Generally, a medical history would need to be examined for other comorbidities, as well as medication history to rule out other possibilities.
There’s usually no concrete testing procedure for Parkinson’s, with imaging tests such as DaTscan being used as a confirmatory test. During a DaTscan a radioactive substance is provided to a patient who is then placed in a single-photon emission computed tomography (SPECT) scanner akin to an MRI. The radioactive substance binds to dopamine transmitters, and thus a reduction of binding as seen through imaging may be indicative of Parkinson’s disease. Research continues to look2 at other imaging methods that can better improve PD diagnosis and confirmation.
In general, an assessment for bradykinesia as well as a tremor of some sort can lead to a diagnosis of PD, with a DaTscan or other imaging methods providing confirmation (or at least helps rule out other possibilities).
Although motor disturbances are the most recognized signs of PD, there are various other signs and symptoms that can manifest with the disease as well, such as:
sleep disturbances
loss of smell
cardiovascular complications
bladder dysfunction/incontinence
gastrointestinal issues
difficulty swallowing or speaking
Most are likely linked to the neurodegeneration. Strangely, some of the early signs of PD may be the loss of smell, so it’s interesting to consider such a symptom within the context of the COVID pandemic—we’ll discuss this further in a later post.
Causes of Parkinson’s
Like with many diseases the cause of PD is multifaceted, and much is still unknown about the main drivers of this disease. In short, the combination of genetic and environmental factors are likely responsible for the progression and pathology of the disease.
One main area of focus for PD relates to deterioration in a region of the midbrain known as the substantia nigra. This region is involved with the production of dopamine as well as other neuronal mechanisms.
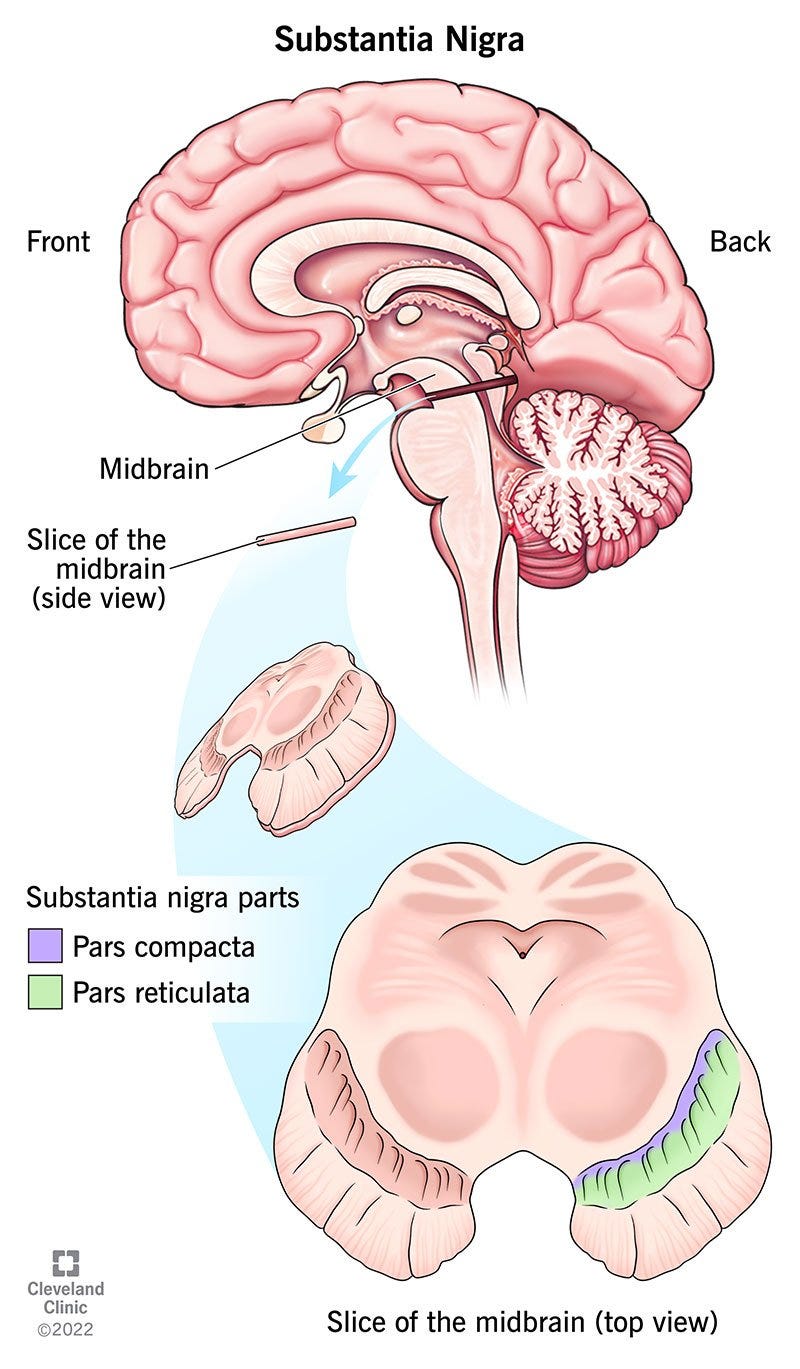
It’s been assumed that deteriorating function within the substantia nigra is one of the main aspects of PD as the loss of dopamine may lead to the motor and muscular dysfunctions presented by the disease. This can be corroborated with the use of one common PD therapeutic levodopa (L-DOPA)3, which acts as a dopamine precursor that crosses the blood-brain barrier and gets converted into dopamine within the central nervous system.
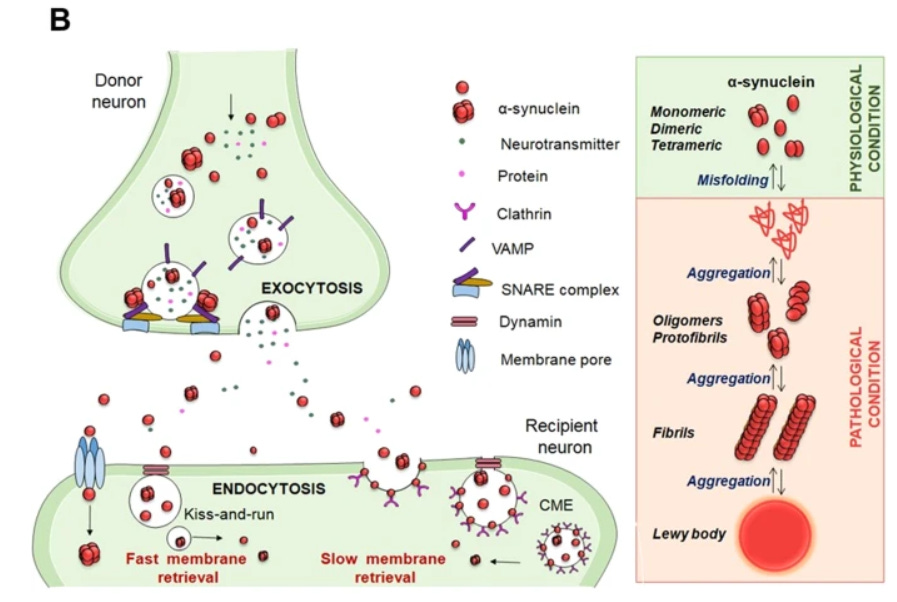
Much of this destruction of the substantia nigra and other neurons appears to come from a specific protein called α-synuclein. α-synuclein is a small acidic protein found within various regions of the nervous system as well as within the blood and various tissues. α-synuclein can be found as an unfolded monomer, although the folded tetramer form has also been recognized.
The unfolded monomer has been implicated as having aggregation properties, such that the monomer can orient in a β-sheet conformation which attracts other monomers that can then form into oligomers. Larger fibril structures can form into what’s known as a Lewy body which can lead to disruption, occlusion, and destruction of neurons and neuronal signaling. These protein aggregates can also progress into what’s known as Lewy body dementia, which is the second most common form of dementia behind Alzheimer’s.
Thus, it is likely a consequence of misfolding maintenance and protein clearance that leads to the accumulation of these Lewy bodies and the progression of PD, with severe damage possibly occurring in the substantia nigra. However, there have been some comments suggesting that the formation of Lewy bodies may not be destructive, and may be a protective method of collecting otherwise harmful misfolded proteins4.
The exact cellular actions of α-synuclein are poorly understood, with a general assumption being that the protein plays a role in synaptic signaling and protein trafficking (Calabresi, et al.5):
A-syn is mainly present at presynaptic sites where it interacts with proteins involved in the release and reuptake of neurotransmitters [24]. Moreover, α-syn is involved in sensing and stabilizing curved membranes [25] and regulating the synaptic vesicle pool and trafficking [26]. In this regard, α-syn has a role in the assembly of the Soluble N-ethylmaleimide Attachment protein REceptor (SNARE) complex, which consists of a set of presynaptic proteins that mediate different phases of the exocytosis process [24] (Fig. 1B).
The role of α-synuclein becomes further complicated given the fact that its role likely changes depending on the cell types and location in which the protein is expressed.
It’s possible that this difference in α-synuclein utilization may be the reason that neurons are selectively targeted by this protein at higher concentrations, and may explain the progression of PD as misfolded α-synuclein may be transported to other neurons, thus leading to the propagation of protein aggregation.
This may explain why the substantia nigra and dopaminergic neurons in particular may be targeted due to the high mitochondrial activity which may put these cells at greater risk of dysfunction:
Accordingly, neurons with extensive axonal arborizations and high requirements for mitochondrial activity, such as DA neurons, may be more susceptible to α-syn aggregation [41]. A-syn plays a direct role in mitochondrial activity [44, 45]. In particular, pathological α-syn mediates functional mitochondrial failure, disruption of mitochondrial morphology [46], impairment of complex I function [47], mitochondria accumulation, and decreased basal mitochondrial oxygen consumption rate [45]. There is also evidence that nitrosative and oxidative stress causes abnormal protein accumulation by impairing the ubiquitin-proteasome system [48], leading to a vicious circle of oxidative cellular damage, toxic α-syn accumulation, and neuronal death. In particular, increased mitochondrial oxidative stress in fibroblast-derived induced pluripotent stem cells, differentiated into human nigral DA neurons, triggers a DA-dependent toxic cascade, leading to oxidized DA accumulation and reduction in glucocerebrosidase enzymatic activity, lysosomal dysfunctions, and α-syn accumulation [49].
Interestingly, a missense mutation in the SNCA gene—the gene that codes for α-synuclein— has been considered one key determinant in PD disposition.
According to Calabresi, et al. this mutation may lead to the accumulation of α-synuclein which activates an immunological response to deal with the proteins. This activation stems from microglial cells, which act as macrophages within the nervous system and may activate an inflammatory response:
A-syn gene (SNCA) missense mutations lead to the accumulation of α-syn and activation of microglia, neuroinflammation, and degeneration of the striatal neurons [58]. Inflammatory mediators, such as reactive oxygen species (ROS), nitric oxide (NO), tumor necrosis factor (TNF)-alpha(α), and interleukin (IL)-1beta(β), derived from non-neuronal cells, are known to modulate the progression of neuronal cell death in PD [59, 60] (Fig. 2).

Several other mechanisms related to PD are still being elucidated, and further research may provide other explanations.
Parkinsonism
Although PD has been looked at closely PD falls into a broader syndrome with characteristics similar to that of PD called parkinsonism.
This categorization encapsulations symptoms of bradykinesia and tremors that may be associated with PD, Lewy body dementia, and other conditions, which can be imagined as a Venn diagram6:
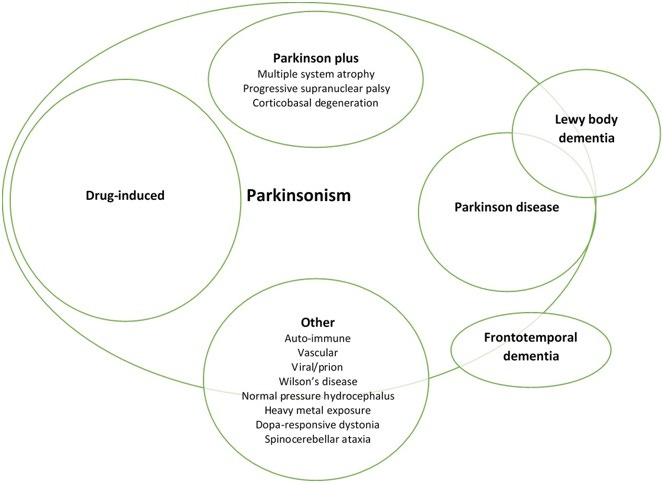
It’s fascinating to consider that multiple factors can influence the expression of parkinsonism. Evidence continues to mount to suggest that viral infections, drugs, toxins, and other environmental circumstances can influence the progression or onset of PD or parkinsonism7.
For instance, various drugs which target dopamine receptors may contribute to drug-induced parkinsonism, which includes antipsychotics and SSRIs (Greenland, J.C. & Barker, R.A.):
Drugs that can act on dopamine receptors in the central nervous system can cause parkinsonism, as well as other movement disorders including tardive dyskinesia, akathisia, and dystonia. Antipsychotics are the most common cause of drug-induced parkinsonism, particularly the “typical antipsychotics” including haloperidol and chlorpromazine, although these drugs are now rarely used in clinical practice. However, atypical antipsychotics such as risperidone and olanzapine can also cause extra-pyramidal side effects, including parkinsonism.
Other causes of drug-induced parkinsonism include anti-emetics (e.g., metoclopramide and domperidone), calcium channel blockers (e.g., flunarizine and cinnarizine), anti-epileptics (e.g., sodium valproate and phenytoin), dopamine depleting drugs (e.g., tetrabenazine), and selective serotonin reuptake inhibitor anti-depressants (30).
As such, those on such medications may need to be closely examined by medical professionals in case of the onset of parkinsonism.
Viral parkinsonism, which will be explained further in the next post, has come into question given findings that have noted a correlation between diagnoses of parkinsonism and prior viral outbreaks, as noted by Jang, et al.8 in their review:
The idea that viruses or other contaminating agents may be an initiating etiology of primary PD or causative for secondary parkinsonism often relates to the findings of coincident cases of parkinsonism that lie outside of the expected. One of the most famous, and still controversial, examples is the parkinsonism that occurred subsequent to a viral encephalopathy that developed following the 1918 influenza pandemic [29].
Another example that suggests viral agents can act as an initiator of parkinsonism is the appearance of what appears to be “parkinsonian clusters”. These are groups of individuals who share common environments and develop parkinsonism at greater than normal statistical rates without the obvious typical risk factors. In fact, the risk factor for developing Parkinson's disease is approximately 2× greater in people who share close quarters, including doctors and nurses, teachers and religion-related jobs. Several of these “parkinsonian clusters” have been described, including those living in Israeli kibbutz's, group of college teachers, garment workers in a manufacturing factory and a group of actors, producers and technical staff working on a television series in Canada [30, 31].
Again, much of the discussion on viral parkinsonism will be saved for the next post.
Treatment for Parkinson’s
Similar to Alzheimer’s most treatments for PD focus on reducing symptoms and help with management of the disease, usually through medications that elevate dopamine levels in the brain such as L-DOPA or aid in prolonging the effects of such drugs.
Techniques such as deep brain stimulation are also an option. However, in recent years the growing trend of examining marijuana and cannabinoid-containing items have lead to extensive research in this field for viable treatment options for neurodegeneration.9
Diet and exercise may also help with the management of PD and parkinsonism, although that should be discussed with medical professionals to see the best option.
Interestingly, as has been the case for many diseases highlighted previously, some research has looked into the role of the microbiome in parkinsonism given the dynamics between the gut-brain axis in health.10
As we deal with a growing elderly population the rate of PD diagnoses will likely rise.
So far, there is still much left to be figured out in detailing what causes PD or how to treat it.
However, what’s important to note is that many factors are likely to influence the onset of PD.
The next post will dive further into viral parkinsonism and taking a look to see if the COVID pandemic may be associated with an increased onset of parkinsonism in the coming years.
Additional Resources:
Consider checking the hyperlinks above for additional information. The few below may also help to supplement the information provided in the previous links.
Panicker, et al.11 : The Cell Biology of Parkinson's Disease
Meade, et al.12: Alpha-synuclein structure and Parkinson’s disease – lessons and emerging principles
Reich, S. G., & Savitt, J. M.13: Parkinson's Disease
Substack is my main source of income and all support helps to support me in my daily life. If you enjoyed this post and other works please consider supporting me through a paid Substack subscription or through my Ko-fi. Any bit helps, and it encourages independent creators and journalists such as myself to provide work outside of the mainstream narrative.

Parkinson J. (2002). An essay on the shaking palsy. 1817. The Journal of neuropsychiatry and clinical neurosciences, 14(2), 223–222. https://doi.org/10.1176/jnp.14.2.223
Feraco, P., Gagliardo, C., La Tona, G., Bruno, E., D'angelo, C., Marrale, M., Del Poggio, A., Malaguti, M. C., Geraci, L., Baschi, R., Petralia, B., Midiri, M., & Monastero, R. (2021). Imaging of Substantia Nigra in Parkinson's Disease: A Narrative Review. Brain sciences, 11(6), 769. https://doi.org/10.3390/brainsci11060769
Gandhi KR, Saadabadi A. Levodopa (L-Dopa) [Updated 2022 May 2]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482140/
There appears to be some debate as to the actual role of Lewy bodies, as autopsy reports of those with diagnosed PD don’t appear to show any indication of Lewy bodies in the brains of these individuals. In contrast, autopsies of people with Lewy body aggregates but no clinical indication of PD when alive has also been noted. This has raised questions as to what degree Lewy bodies may serve as pathological “bystanders”, adding to the overall complexities of neurodegenerative diseases.
Chartier, S., & Duyckaerts, C. (2018). Is Lewy pathology in the human nervous system chiefly an indicator of neuronal protection or of toxicity?. Cell and tissue research, 373(1), 149–160. https://doi.org/10.1007/s00441-018-2854-6
Interestingly, Brian Mowrey raised some ideas towards a protective role of amyloids with respect to the amyloid hysteria surrounding COVID spike. Although not pertinent to the discussion, it’s interesting to see this concept of toxic/protective roles of protein aggregates being discussed:
Calabresi, P., Mechelli, A., Natale, G. et al. Alpha-synuclein in Parkinson’s disease and other synucleinopathies: from overt neurodegeneration back to early synaptic dysfunction. Cell Death Dis 14, 176 (2023). https://doi.org/10.1038/s41419-023-05672-9
Cook Shukla L, Schulze J, Farlow J, et al. Parkinson Disease Overview. 2004 May 25 [Updated 2019 Jul 25]. In: Adam MP, Everman DB, Mirzaa GM, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1223/
Greenland JC, Barker RA. The Differential Diagnosis of Parkinson’s Disease. In: Stoker TB, Greenland JC, editors. Parkinson’s Disease: Pathogenesis and Clinical Aspects [Internet]. Brisbane (AU): Codon Publications; 2018 Dec 21. Chapter 6. Available from: https://www.ncbi.nlm.nih.gov/books/NBK536715/ doi: 10.15586/codonpublications.parkinsonsdisease.2018.ch6
Jang, H., Boltz, D. A., Webster, R. G., & Smeyne, R. J. (2009). Viral parkinsonism. Biochimica et biophysica acta, 1792(7), 714–721. https://doi.org/10.1016/j.bbadis.2008.08.001
Cooray, R., Gupta, V., & Suphioglu, C. (2020). Current Aspects of the Endocannabinoid System and Targeted THC and CBD Phytocannabinoids as Potential Therapeutics for Parkinson's and Alzheimer's Diseases: a Review. Molecular neurobiology, 57(11), 4878–4890. https://doi.org/10.1007/s12035-020-02054-6
Gazerani P. (2019). Probiotics for Parkinson's Disease. International journal of molecular sciences, 20(17), 4121. https://doi.org/10.3390/ijms20174121
Panicker, N., Ge, P., Dawson, V. L., & Dawson, T. M. (2021). The cell biology of Parkinson's disease. The Journal of cell biology, 220(4), e202012095. https://doi.org/10.1083/jcb.202012095
Meade, R.M., Fairlie, D.P. & Mason, J.M. Alpha-synuclein structure and Parkinson’s disease – lessons and emerging principles. Mol Neurodegeneration 14, 29 (2019). https://doi.org/10.1186/s13024-019-0329-1
Reich, S. G., & Savitt, J. M. (2019). Parkinson's Disease. The Medical clinics of North America, 103(2), 337–350. https://doi.org/10.1016/j.mcna.2018.10.014



pesticides are associated with it as well.. paraquat is one.... i wonder how common it is in more pristine places and if there was an increase in developing nations when they were pushed to use chemicals from the west
thank you for the link to unglossed article!! theres a pretty popular substack that seems to enjoy stirring up doom and gloom re the spike whether vaxxed or not and my queries as to proof of whether other viruses do these same things has never been answered. now i can repost unglossed to calm the hysteria... lol...