COVID Vaccines and Parkinson's Disease Part III-2
What effect did the COVID pandemic have on neurodegeneration; and does SARS-COV2 actually penetrate the brain?
In Consideration of COVID-19 and the Braak Hypothesis
In our previous post (Part III-1) we looked at one explanation (the Braak hypothesis) as a pathogen-related cause for Parkinson’s and other neurodegenerative diseases.
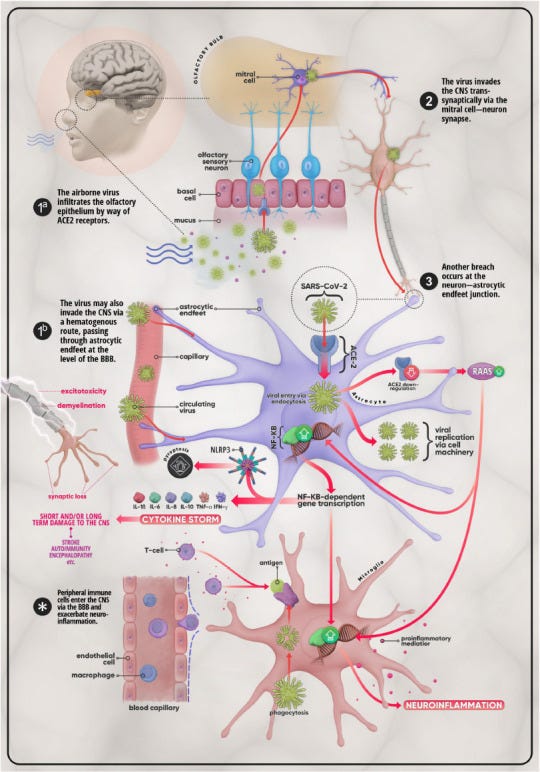
Fascinatingly, the Braak hypothesis points towards the susceptibility of nasal neurons to invasion from viruses, and thus may serve as one route in which the central nervous system gets targeted via the virus taking advantage of the weaker blood-brain barrier dynamics within the nose (and possibly the enteric system) relative to the rest of the body.
Such a proposed model can be seen in a review from Haidar, et al.1:
Several pieces of evidence with respect to SARS-COV2, when paired with the Braak hypothesis, may raise serious concerns as to whether the virus is directly targeting the central nervous system and may be causing damage to the brain in many who have been infected, leading to possible long-term damage as well as early onset Alzheimer’s and Parkinson’s disease.
This is also paired with the concerns that those already predisposed to neurodegenerative diseases may have symptoms of neurodegeneration become unmasked due to the pandemic. Those who are already diagnosed with Alzheimer’s, dementia, or Parkinson’s disease may be more at risk of even worse symptoms.
This may also explain an overlap of long COVID symptoms with those of parkinsonism, with symptoms possibly lasting for quite a long time and may be detrimental to long-term health.
The literature is filled with articles raising questions as to whether diagnoses of parkinsonism will increase in the coming years, with a few raising concerns in those with PD by noting an increase in motor dysfunction and levodopa prescription in those with PD who also contracted COVID, suggesting that long-COVID in these individuals may be an exacerbation of Parkinson’s-like symptoms.
Given the scope of this article I leave various sources to take a look at for those interested:
Rai, et al.2: Exploring the Paradox of COVID-19 in Neurological Complications with Emphasis on Parkinson's and Alzheimer's Disease.
van der Heide, et al.3: The Impact of the COVID-19 Pandemic on Psychological Distress, Physical Activity, and Symptom Severity in Parkinson's Disease.
It’s important to note that not all of the worsening symptoms of Parkinson’s disease is derived from direct viral infection. Instead, there’s a good argument to be made that the lockdown policies, which reduced mobility and interactions with loved ones, also contributed to the worsening of symptoms.
Leta, et al, 20214: Parkinson's Disease and Post-COVID-19 Syndrome: The Parkinson's Long-COVID Spectrum.
This review noted a decline in motor function as well as an increase in levodopa dosage in this case series of those with Parkinson’s disease who were infected with COVID.
Leta, et al, 20225: Covid-19 and Parkinson's disease: Acute clinical implications, long-COVID and post-COVID-19 parkinsonism
This review article has similar authors as the previous article, and provides this interpretation of various mechanisms of worse parkinsonism due to the pandemic
Rosen, et al.6: The Intersection of Parkinson's Disease, Viral Infections, and COVID-19.
This article makes an association, albeit limited, between SARS-COV2 and the Braak hypothesis.
Goerttler, et al.7: SARS-CoV-2, COVID-19 and Parkinson's Disease-Many Issues Need to Be Clarified-A Critical Review.
This review mentions a few case reports of new-onset parkinsonism following a SARS-COV2 infection.
Kukkle, P. L.8: COVID-19: The cynosure of rise of Parkinson's disease.
Eldeeb, et al.9: COVID-19 infection may increase the risk of parkinsonism - Remember the Spanish flu?
This article, as well as the prior one, raise concerns towards a possible increase in parkinsonism diagnoses following the pandemic.
Cartella, et al.10: Covid-19 and Parkinson's disease: an overview.
Merello, et al.11: SARS-CoV-2 and the risk of Parkinson's disease: facts and fantasy.
Ali, et al.12: New-onset Parkinsonism as a Covid-19 infection sequela: A systematic review and meta-analysis.
Indeed, the commonly present sign of anosmia (loss of smell) from even a minor COVID infection appears to mimic the initial symptoms of parkinsonism, adding credence to the Braak hypothesis in particular with SARS-COV2. This is paired with reports of brain fog, confusion, and other neuropsychological symptoms13 in many who have been infected with SARS-COV2.
Now, this raises various questions towards what mechanisms could possibly be causing these symptoms.
This includes considering whether SARS-COV2 is a neuroinvasive virus that displays neurotropism. These terms sound familiar, but they should not be used synonymously:
Neuroinvasive: a pathogen that can readily invade the nervous system of an organism.
Neurotropism: a pathogen that readily gets taken up and replicated by cells (i.e. replication-competent) within the nervous system.
Many infectious agents may be able to cross the blood-brain barrier (neuroinvade), but that doesn’t mean that those agents can readily multiply in that region of the body (neurotropic).
In the Dr. Rudy Tanzi interview provided by Brian Mowrey of Unglossed Tanzi suggests that SARS-COV2 shows neuroinvasive properties yet neurotropism appears to be limited. That is, even if the virus can make its way into the CNS it does not appear that the virus can continue to replicate within this region.
This is also corroborated in a review from Bauer, et al.14 which I encourage people to read given the completeness and the images provided in this review. Not only does Bauer, et al. review the neuroinvasive and neurotropic behaviors of SARS-COV2, but also examines another topic of neurovirulence, in which the virus may manifest neurological complications without having direct involvement with the nervous system.
But again, the remarks from Bauer, et al. appear to parallel that of Tanzi:
Currently available data show that SARS-CoV-2 has neuroinvasive potential, that its neurotropism is limited, and that it can be neurovirulent in at least a subgroup of patients. This concurs with observations from the clinic, where the impact of SARS-CoV-2-associated CNS complications appears limited during the acute phase, but more prominent during the postacute phase. Reports of severe disease during the acute phase, such as encephalitis, exist, but these are rare compared with the number of people infected [92,93]. However, the percentage of patients with SARS-CoV-2-associated CNS impairments during the postacute phase, which are part of the wide spectrum of complications associated with long COVID, can be up to 30–60% [5., 6., 7.].
In some ways these remarks may assuage concerns that SARS-COV2 continuously erodes the brain through destruction of neurons. However, remember that foreign agents that make their way into the brain may still trigger neuroinflammation and the formation of amyloid plaques, Lewy body aggregates, as well as other antimicrobial peptides associated with neurodegeneration.
The fact that neurinvasion of SARS-COV2 may be possible would still suggest the formation of these proteins, which would still be concerning.
Thus, evidence of SARS-COV2 being capable of passing the blood-brain barrier would still warrant some concern.
In addition, what remains to be seen is whether the pandemic may lead to more diagnoses of Parkinson’s disease, dementia, or other neurodegenerative diseases in the coming years.
SARS-COV2 Neuroinvasion in Animal Models
Several lines of in silico studies, as well as various animal models have examined the possibility of neuroinvasion.
For instance, a study Rhea, et al. in 2020 proposed that the S1 subunit of SARS-COV2 can readily pass across the blood-brain barrier in mice when administered intravenously. The following looked at two commercially available S1 subunits and compared to T-Alb uptake (control):

However, an in vitro assessment using human cell lines (human induced pluripotent stem cell (iPSC)-derived brain endothelial-like cells (iBECs)) to mimic the human blood-brain barrier did not find similar results, with findings comparable to that of the Albumin15 controls:

This discrepancy in findings could be due to factors related to in vitro testing, or it may be indicative of inter-species differences in blood-brain barrier construction. It’s been remarked that many drugs intended to target the CNS do not translate well from animal models into human models due to species differences in BBB makeup, and thus could be an influence here as well.16
In a monkey study from Ingrid, et al.17 using both rhesus macaques (n=4) and cynomolgus macaques (n=4) the animals were challenged with both intratracheal and intranasal SARS-COV2. In the following weeks the monkeys were sacrificed and autopsied for evidence of SARS-COV2 in the brain and nose of infected monkeys.
Relative to controls, the infected macaques showed signs of T-cell and microglial activation in the brain18. Interestingly, the infected monkeys also showed signs of Lewy body aggregates.
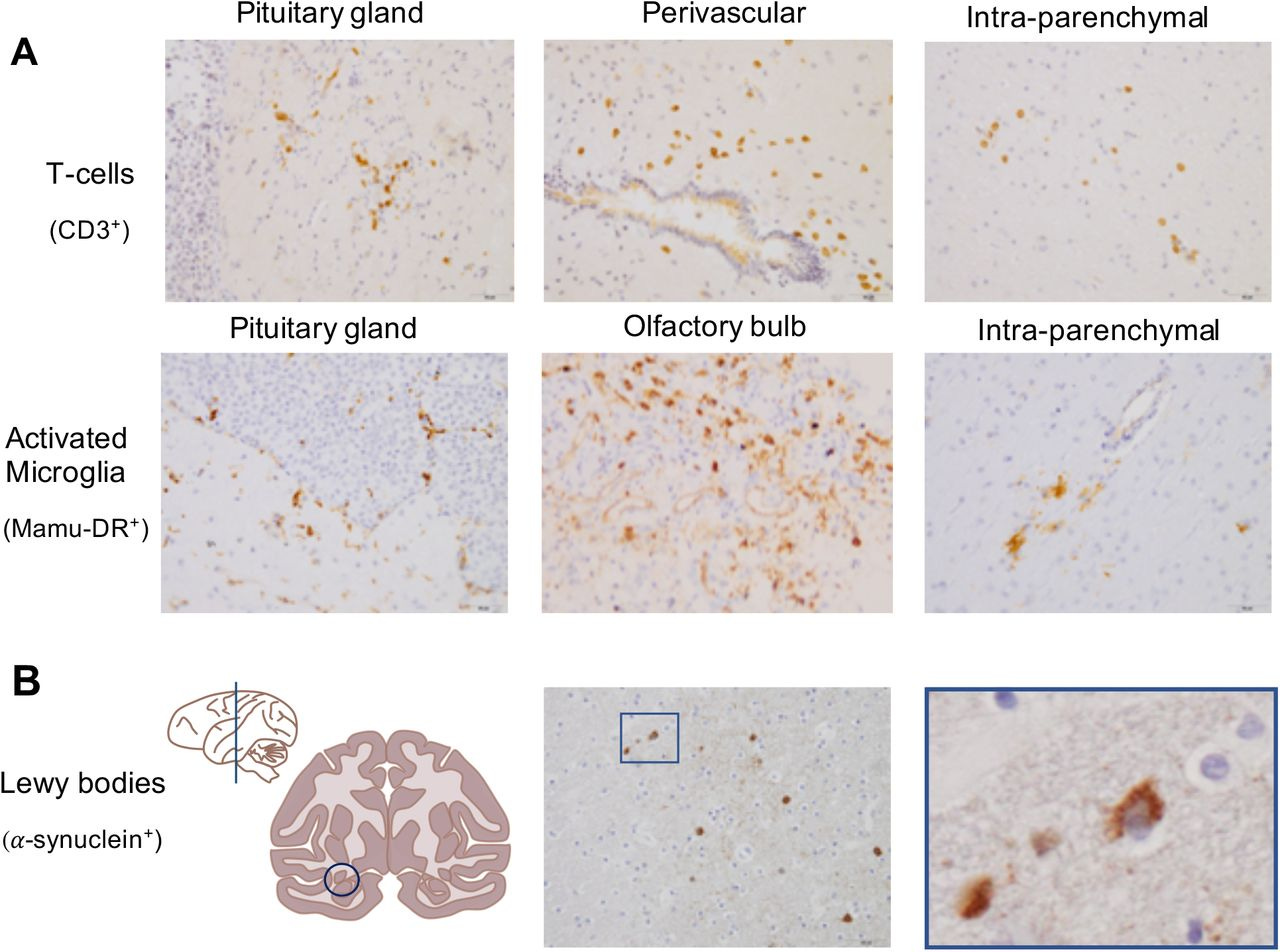
These findings add more assurance to the idea that the brain can be targeted by SARS-COV2 given the presence of the immunological markers, as well as the Lewy bodies that were not seen in the brains of control monkeys.
Here, the researchers make the following conclusion:
There is a growing concern that symptomatic COVID-19 patients may suffer from long-term consequences (9, 38). In this light the finding of Lewy bodies in brains of infected macaques without overt clinical signs is intriguing. Together with signs of inflammation and immune activation in the brains of the macaques this finding may point to a not yet earlier described SARS-CoV-2-induced neurodegenerative process that can explain the neurological symptoms that COVID-19 survivors experience (39).
Lewy bodies are considered a hallmark for the development of Parkinson’s disease, or Lewy body dementia. More confirmation is required, but the observations in the translational macaque models for COVID-19 (12-14, 40, 41) can be regarded as a serious warning as they may be predictive for COVID-19-related dementia cases in humans in the future, even after an asymptomatic infection or mild disease process.
But as we have been previously made aware, and remarked on by Tanzi in his video, the presence of the Lewy bodies themselves are not inherently indicative of neurodegeneration. They can, in fact, be representative of a neuroprotective mechanism, in which case the Lewy bodies may have aggregated around virions and viral debris to prevent cytotoxic damage within the sensitive areas of the brain.
It’s once again the issue in which an observed biomarker is considered to play a causative role in a disease’s pathology when the presence itself may be pointing towards something more beneficial/protective of the body.19
Interestingly, the researchers don’t note much about behavioral changes within these monkeys. They mention a lack of “overt clinical signs” in the excerpt above, but there’s no indication to what that means in particular, which doesn’t tell us whether the infection and presentation of Lewy bodies may have been associated with behavioral changes in the macaques. Again, remember that the presence of Lewy bodies themselves are not indicative of symptoms of parkinsonism.
But given these findings is there enough here to warrant an argument in favor of neuroinvasion and the Braak hypothesis at play? Are many of us doomed to the formation of amyloid plaques and Lewy bodies in the brain-a concern raised by Tanzi himself given his two bouts with COVID?
Surprisingly, this may be a place where consensus around COVID may actually deviate.
Does spike actually get into the brain?
So far I haven’t discussed much within the realms of the blood-brain barrier, which I may save for a supplemental reading.
In short, normally the body separates the blood from other tissues via the endothelial layer of cells that make up blood vessels. This layer provides a barrier of exchange between the local tissue environment and nutrients/cells within the blood.
However, because the central nervous system is extremely delicate the exchange of nutrients and cells between the blood and the CNS are more tightly regulated. This more regulated feature is what’s referred to as the blood-brain barrier. So tightly regulated is this feature that many pathogens, nutrients, and even drugs find difficulty passing between the blood and the interstitial space/fluid within the CNS.
Thus, given this difficulty in crossing the BBB one may wonder how pathogens can still make it across into the brain and spinal chord. This is all dependent upon size, polarity, and receptor-mediated transport.
However, in many cases, such as in those with neurodegenerative disease, the BBB becomes extremely permeable, thus allowing things to pass more freely between the blood and the interstitial space.
This aspect of cerebrovascular damage and BBB permeability has gained traction in recent years, and may be one main factor in disease progression20, being called a possible vascular arm of neurodegeneration.
This may seem unimportant, but let’s consider the argument that SARS-COV2 exhibits neuroinvasive properties. Wouldn’t we, in essence, consider that neuroninvasion would be far more likely in those with permeable BBB via neurodegeneration, and serve as a good model for “spike in the brain?”.
A few months ago a study was published by Nuovo, et al.21 looking at autopsy data from people with dementia who were infected with SARS-COV2 (n=5).
The information was compared to those who died of COVID without any indication of dementia (n=8), those who died of Alzheimer’s prior to COVID (n=10) and age-matched controls (n=10).
Although a rather limited size the information here would be considered invaluable. As stated above, those with dementia should, hypothetically, show evidence of spike penetrating within the brain of these individuals.
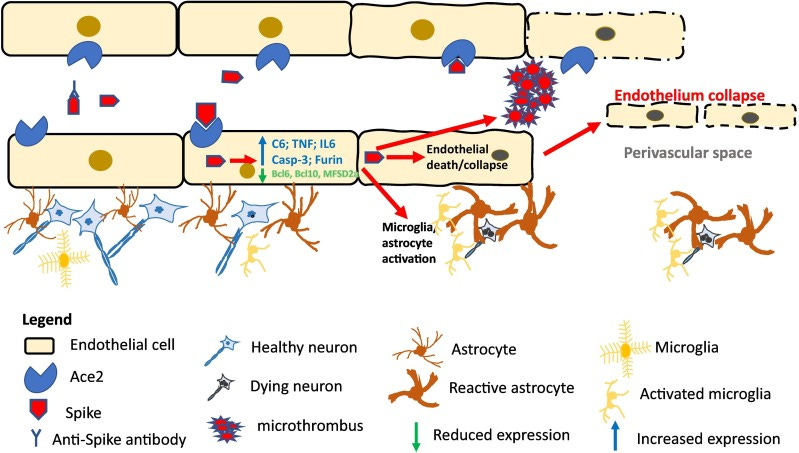
Instead, it appears that in those with SARS-COV2 infections the primary driver of neuropathologies may be the uptake of spike by endothelial cells and neuroinflammation without direct invasion of the virus (at least in this study).
*This review was rather description so I unfortunately have taken several excerpts since the histology slides were not sufficient for the discussion. Skip ahead for a summary of what all of the information means in context.
When looking at viral RNA as well as the presence of nucleocapsid proteins in the brain of COVID-infected patients (both with and without dementia) there was hardly any detection of these material unlike the presence of spike protein’s subunits:
SARS-CoV2 RNA was evident in only 2/13 COVID-19 brain samples (0/5 dementia/COVID-19 cases) and in rare cells in the two positive cases, despite documenting that the lung tissues from most of these people had very high copy SARS-CoV2 RNA (Fig. 2 ). Serial sections of the controls and the COVID-19 cases were then analyzed for the viral spike proteins (subunits 1 and 2, (S1, S2)) and the nucleocapsid protein in a blinded fashion. The nucleocapsid protein was only detected, and rarely, in 2/13 COVID-19 cases (0/5 COVID-19/dementia cases) and none of the controls; these were the same two cases with rare viral RNA. Contrary to the viral RNA and the nucleocapsid, spike S1 and S2 subunits were abundant in the COVID-19 cases and not evident in the controls.
And a comparison of control tissues to COVID-19 patients noted that various pro-inflammatory and pro-coagulant markers were elevated in those with COVID-19:
Both the COVID-19 cases with no pre-existing dementia and the COVID-19 cases with pre-existing Alzheimer's disease showed equivalent findings: there was strong expression of Caspase-3, IL6, TNFα, CD41 and Complement component 6 that showed the same distribution as spike S1 and S2. Specifically, the percentage of microvessels with at least one cell that stained positive for Caspase-3, IL6, TNFα, or Complement component 6 ranged from 18.9 to 27.9 (mean 22.2) in the COVID-19 non-dementia cases and 17.8 to 29.9 (mean 24.0) in the COVID-19/Alzheimer's disease cases which was statistically equivalent. Co-expression experiments documented a strong co-localization of each of the mentioned proteins with spike S1 (Fig. 3); note the strong association of the protein co-localized with S1 with microvascular damage.
Interestingly, in those with Alzheimer’s dementia the presence of endocytosed spike was nearby regions of the brain with hyperphosphorylated tau proteins (an indication of Alzheimer’s), although this may just be coincidental given the small sample data:
Thus, we quantified the spike protein detection in the areas of the brain with abundant hyperphosphorylated tau protein and compared this to areas with little to no hyperphosphorylated tau protein in the Alzheimer's disease cases. Although there was a tendency for higher spike/cytokine density in the hyperphosphorylated tau protein + areas, it did not reach statistical significance (spike density in areas that stained positive for hyperphosphorylated tau protein was 25.5 % of microvessels with SEM of 5.1 versus 22.9 % of microvessels with SEM of 6.1 in sections with minimal hyperphosphorylated tau protein).
And lastly, proteins associated with Alzheimer’s were found to have been disturbed in those with SARS-COV2 compared to controls (lower levels were associated with Alzheimer’s and other dementias), suggesting that dysfunction of these proteins via spike endocytosis may contribute to exacerbated neurological symptoms in patients SARS-COV2:
The COVID-19 brains with no history of dementia showed a marked reduction (>50 %) in the expression of BCL6, BCL10 (Fig. 4), BACH1, SHIP1, and MFSD2a with no statistically significant difference in the COVID-19 brain tissues from people who had pre-existing Alzheimer's disease. Similarly, there was a marked increase in the expression of nNOS, NMDAR2, and Furin in the brain tissues of people who died of COVID-19 with equivalent results in the group with no history of dementia and in the group with pre-existing Alzheimer's disease. As evident from Fig. 4, the nNOS, NMDAR2, and Furin showed a strong co-localization with the endocytosed spike protein (Fig. 4).
In Summary…
There’s a ton of text here, but the gist of this study presents something counter to direct neuroinvasion, but rather that spike protein may be uptaken by endothelial cells and thus leads to the alterations in cellular function as well as neuroinflammation via microglial activation:
The data in this paper supports the hypothesis that the endocytosis of circulating spike protein in the ACE-2-expressing endothelial cells of microvessels in the CNS plays a major role in the CNS disease of COVID-19. This is not to say that other factors, such as the cytokine storm and antibody-antigen complexes in the microvessels, do not also play a role. However, it is now clear that endocytosed spike protein in the microvessels of the CNS or in vitro can induce the pro-inflammatory microenvironment and hypercoagulable state well described in the brain for fatal COVID-19 before a systemic cytokine storm or antibody response would be mounted [12], [15], [17].
As reported, the responding immune cells in the brains of these COVID-infected individuals were predominately microglial cells and astrocytes, suggesting that immune activation was dictated by cells localized within the CNS rather than exogenous immune cells that may pass from the blood into the brain via the BBB (which counters the outline from Haider, et al. shown at the beginning of the post). This was noted in in vitro studies which suggested microglia activation by spike as a mechanism of neuroinflammation and a possible explanation for long COVID symptoms.22
Therefore, it’s possible that this microglial activation rather than direct neuroinvasion may be responsible for the neuroinflammation.
In support of this assumption Nuovo, et al. proposes that microglial cells already stressed by dealing with tau tangles and Lewy bodies may be further stressed, likely leading to oxidative stress of cells and other deleterious consequences. This also comes as some of the proteins expressed by endothelial cells, many of which are involved with maintaining the BBB, become less expressed, thus making the BBB more susceptible, and may be one process responsible for the progression of dementia.
But strangely, above all these findings may point to something that many people have suggested— that SARS-COV2 may be thought of more as a vascular disease. It’s only through the specific region of this vascular dysfunction do the symptoms present in such a unique manner.
Note that this hypothesis doesn’t preclude any other hypotheses out there—it’s possible to consider that neuroinvasion still occurs and may be part of the reason for neurological impairments and long COVID. Rather, it does raise questions as to whether the prevailing assumption with respect to SARS-COV2 may have been incorrect under certain circumstances. As stated before, one would assume that those with Alzheimer’s and Lewy body dementia may be more inclined to have a more permeable BBB and thus more translocation of spike and other viral debris into the brain, which doesn’t appear to be the case here.
So maybe the Braak hypothesis may not hold up as well as initially thought, although it’s still an interesting concept to consider in light of other hypotheses. Further research, which as of now is rather limited, may add additional evidence in either direction of actual neuroinvasion of the virus or of endothelial dysfunction as being a main driver of neurological symptoms.
Now, given all of this information that leaves us with the critical issue of the case report from Mörz, M.23. Given what we've found could we provide more context to this case report and consider whether the events and exacerbation of Parkinson's disease may be due to the vaccine?
Substack is my main source of income and all support helps to support me in my daily life. If you enjoyed this post and other works please consider supporting me through a paid Substack subscription or through my Ko-fi. Any bit helps, and it encourages independent creators and journalists such as myself to provide work outside of the mainstream narrative.

Haidar, M. A., Shakkour, Z., Reslan, M. A., Al-Haj, N., Chamoun, P., Habashy, K., Kaafarani, H., Shahjouei, S., Farran, S. H., Shaito, A., Saba, E. S., Badran, B., Sabra, M., Kobeissy, F., & Bizri, M. (2022). SARS-CoV-2 involvement in central nervous system tissue damage. Neural regeneration research, 17(6), 1228–1239. https://doi.org/10.4103/1673-5374.327323
Rai, S. N., Tiwari, N., Singh, P., Singh, A. K., Mishra, D., Imran, M., Singh, S., Hooshmandi, E., Vamanu, E., Singh, S. K., & Singh, M. P. (2022). Exploring the Paradox of COVID-19 in Neurological Complications with Emphasis on Parkinson's and Alzheimer's Disease. Oxidative medicine and cellular longevity, 2022, 3012778. https://doi.org/10.1155/2022/3012778
van der Heide, A., Meinders, M. J., Bloem, B. R., & Helmich, R. C. (2020). The Impact of the COVID-19 Pandemic on Psychological Distress, Physical Activity, and Symptom Severity in Parkinson's Disease. Journal of Parkinson's disease, 10(4), 1355–1364. https://doi.org/10.3233/JPD-202251
Leta, V., Rodríguez-Violante, M., Abundes, A., Rukavina, K., Teo, J. T., Falup-Pecurariu, C., Irincu, L., Rota, S., Bhidayasiri, R., Storch, A., Odin, P., Antonini, A., & Ray Chaudhuri, K. (2021). Parkinson's Disease and Post-COVID-19 Syndrome: The Parkinson's Long-COVID Spectrum. Movement disorders : official journal of the Movement Disorder Society, 36(6), 1287–1289. https://doi.org/10.1002/mds.28622
Leta, V., Boura, I., van Wamelen, D. J., Rodriguez-Violante, M., Antonini, A., & Chaudhuri, K. R. (2022). Covid-19 and Parkinson's disease: Acute clinical implications, long-COVID and post-COVID-19 parkinsonism. International review of neurobiology, 165, 63–89. https://doi.org/10.1016/bs.irn.2022.04.004
Rosen, B., Kurtishi, A., Vazquez-Jimenez, G. R., & Møller, S. G. (2021). The Intersection of Parkinson's Disease, Viral Infections, and COVID-19. Molecular neurobiology, 58(9), 4477–4486. https://doi.org/10.1007/s12035-021-02408-8
Goerttler, T., Kwon, E. H., Fleischer, M., Stettner, M., Tönges, L., & Klebe, S. (2022). SARS-CoV-2, COVID-19 and Parkinson's Disease-Many Issues Need to Be Clarified-A Critical Review. Brain sciences, 12(4), 456. https://doi.org/10.3390/brainsci12040456
Kukkle P. L. (2022). COVID-19: The cynosure of rise of Parkinson's disease. International review of neurobiology, 165, 251–262. https://doi.org/10.1016/bs.irn.2022.06.007
Eldeeb, M. A., Hussain, F. S., & Siddiqi, Z. A. (2020). COVID-19 infection may increase the risk of parkinsonism - Remember the Spanish flu?. Cytokine & growth factor reviews, 54, 6–7. https://doi.org/10.1016/j.cytogfr.2020.06.009
Cartella, S. M., Terranova, C., Rizzo, V., Quartarone, A., & Girlanda, P. (2021). Covid-19 and Parkinson's disease: an overview. Journal of neurology, 268(12), 4415–4421. https://doi.org/10.1007/s00415-021-10721-4
Merello, M., Bhatia, K. P., & Obeso, J. A. (2021). SARS-CoV-2 and the risk of Parkinson's disease: facts and fantasy. The Lancet. Neurology, 20(2), 94–95. https://doi.org/10.1016/S1474-4422(20)30442-7
Ali, S. S., Mumtaz, A., Qamar, M. A., Tebha, S. S., Parhin, A., Butt, M., & Essar, M. Y. (2022). New-onset Parkinsonism as a Covid-19 infection sequela: A systematic review and meta-analysis. Annals of medicine and surgery (2012), 80, 104281. https://doi.org/10.1016/j.amsu.2022.104281
Serrano-Castro, P.J., Garzón-Maldonado, F.J., Casado-Naranjo, I. et al. The cognitive and psychiatric subacute impairment in severe Covid-19. Sci Rep 12, 3563 (2022). https://doi.org/10.1038/s41598-022-07559-9
Bauer, L., Laksono, B. M., de Vrij, F. M. S., Kushner, S. A., Harschnitz, O., & van Riel, D. (2022). The neuroinvasiveness, neurotropism, and neurovirulence of SARS-CoV-2. Trends in neurosciences, 45(5), 358–368. https://doi.org/10.1016/j.tins.2022.02.006
Albumin is a large protein and therefore does not readily cross the blood-brain barrier. This protein has been utilized in various studies examining the permeability of the BBB under specific conditions due to this fact.
Deo, A. K., Theil, F. P., & Nicolas, J. M. (2013). Confounding parameters in preclinical assessment of blood-brain barrier permeation: an overview with emphasis on species differences and effect of disease states. Molecular pharmaceutics, 10(5), 1581–1595. https://doi.org/10.1021/mp300570z
SARS-CoV-2 causes brain inflammation and induces Lewy body formation in macaques
Ingrid H.C.H.M. Philippens, Kinga P. Böszörményi, Jacqueline A. Wubben, Zahra C. Fagrouch, Nikki van Driel, Amber Q. Mayenburg, Diana Lozovagia, Eva Roos, Bernadette Schurink, Marianna Bugiani, Ronald E. Bontrop, Jinte Middeldorp, Willy M. Bogers, Lioe-Fee de Geus-Oei, Jan A.M. Langermans, Marieke A. Stammes, Babs E. Verstrepen, Ernst J. Verschoor
bioRxiv 2021.02.23.432474; doi: https://doi.org/10.1101/2021.02.23.432474
Chartier, S., & Duyckaerts, C. (2018). Is Lewy pathology in the human nervous system chiefly an indicator of neuronal protection or of toxicity?. Cell and tissue research, 373(1), 149–160. https://doi.org/10.1007/s00441-018-2854-6
Sweeney, M. D., Sagare, A. P., & Zlokovic, B. V. (2018). Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nature reviews. Neurology, 14(3), 133–150. https://doi.org/10.1038/nrneurol.2017.188
Nuovo, G. J., Suster, D., Sawant, D., Mishra, A., Michaille, J. J., & Tili, E. (2022). The amplification of CNS damage in Alzheimer's disease due to SARS-CoV2 infection. Annals of diagnostic pathology, 61, 152057. https://doi.org/10.1016/j.anndiagpath.2022.152057
Frank, M. G., Nguyen, K. H., Ball, J. B., Hopkins, S., Kelley, T., Baratta, M. V., Fleshner, M., & Maier, S. F. (2022). SARS-CoV-2 spike S1 subunit induces neuroinflammatory, microglial and behavioral sickness responses: Evidence of PAMP-like properties. Brain, behavior, and immunity, 100, 267–277. https://doi.org/10.1016/j.bbi.2021.12.007
Mörz M. (2022). A Case Report: Multifocal Necrotizing Encephalitis and Myocarditis after BNT162b2 mRNA Vaccination against COVID-19. Vaccines, 10(10), 1651. https://doi.org/10.3390/vaccines10101651



Thank you, this is so helpful. 🙏💕 I thought covid had the ability to get into the central nervous system because of the loss of smell. I experienced this with omicron, it was very strange. But I didn't notice any change in my ability to taste, which may point to different, more central neural pathways for each of those different sensory capacities (taste and smell). It's helpful to know that the virus doesn't replicate much in the central nervous system.
I understanding is this vaccine injection contains up to a billion nanoparticles, and there are unfortunate people who randomly end up with a lot of nanoparticles and/or synthetic spike protein in their central nervous system.
That's such a brain full of research and info. Thank you every time!