COVID Vaccines and Parkinson's Disease Part III-1
Viral parkinsonism, the Braak hypothesis, and are Lewy bodies harmful or protective?
Edit 3/13/2023: The sub-header stated “Lew” rather than “Lewy”. A correction has been made for the missing -y.
In Part II we discussed one of the main explanations for Parkinson’s Disease through the formation and accumulation of misfolded alpha-synuclein.
Although various causes of parkinsonism was discussed, the main focus on this post will be an examination of parkinsonism related to viral infections.
Viruses and their relation to Parkinsonism
Now, viral infections may seem like a rather strange cause of parkinsonism given that we encounter viruses all the time. However, evidence within the literature detail several instances in which a viral outbreak was proceeded by increased rates of parkinsonism, as previously mentioned in the Jang, et al. review.
One of the most well-recognized incidences of widespread parkinsonism came after the 1918 Spanish Flu outbreak, which interestingly came a year after strange cases of what would be called encephalitis lethargica began to arise and was noted by a Dr. Constantin von Economo1 who named the disease (Hoffman, L. A., & Vilensky, J. A.2):
In late 1916, while treating patients in the Psychiatric Neurological Clinic of the University of Vienna, Dr Constantin von Economo examined several patients who presented with unusual neurological symptoms. The patients had been admitted with varying diagnoses such as meningitis, multiple sclerosis, and delirium; however, none of them matched well into any known diagnostic scheme. In particular, many of the patients presented with marked lethargy. Von Economo considered the uniqueness of this set of symptoms as a distinct disease entity, which he described in a 1917 manuscript entitled Encephalitis Lethargica.
The onset of this strange ailment lasted for nearly a decade, overlapping with the 1918 Spanish Flu outbreak. Patients with encephalitis lethargica would present with flu-like symptoms, parkinsonism, and sleep disturbances, with chronic illnesses of post encephalitic parkinsonism appearing months or even years in the future, with even one recorded case occurring in an individual 45 years after the initial onset of encephalitis lethargica.
There’s no clear number for how many people became afflicted with encephalitis lethargica, but it’s assumed that over a million people may have been afflicted. More interestingly, it’s been assumed that nearly 50% of those afflicted with encephalitis lethargica became afflicted with post-encephalitis parkinsonism, although some numbers have even estimated 80% post-encephalitis parkinsonism.
The actual cause of this encephalitis outbreak is still undetermined. However, given the time period of its emergence and the eventual Spanish Flu outbreak in 1918 it’s been suggested that the disease may have been caused by a virus, although some arguments have been made against this hypothesis3.
Nonetheless, in the years following the Spanish Flu outbreak, caused by the Influenza A subtype H1N1 virus, various cases of neurological impairments began to emerge, becoming one of the most well-known cases of a virus being associated with neurological impairments and parkinsonism.
Henry, et al.4 provides a collection of reports at the time of the viral outbreak, with some of these accounts noting that the relationship between the outbreak and the onset of neurological impairments being more than coincidental:
The most famous influenza pandemic, the one to which all other pandemics have been compared, was the pandemic of 1918, also known as the Spanish flu [1, 4-5]. An estimated 500 million people worldwide were infected between 1918 - 1919, and thus there were many opportunities to observe its neurological manifestations [6].
The British Royal Society of Medicine published their Discussion on Influenza in 1919 [1-2, 5, 7]. This work included discussions on the neurological illness and its apparent relationship to the pandemic. E.B. Turner related his experience with the influenza epidemics of the late 19th century [5]. He postulated that influenza, “attacked more particularly the nerve centres” based on the “very pronounced nerve sequelae observed.” He observed many cases of depression, neurasthenia, neuritis, “and other ills which could only be described as ‘nervous’” in patients recovering from purported influenza infections.
In the discussion of his findings, W. H. Hamer, argued that the temporal relationship between outbreaks of influenza and cerebrospinal fever (meningitis), poliomyelitis, and polio-encephalitis were more than coincidence [7]. He cited his own personal observations from the period between 1914 and 1918. He observed that, although rare, neurological manifestations appeared shortly following the outbreaks of influenza infection.
There have even been evidence to suggest that those born within the timeframe of the EL and Spanish Flu outbreak were at increased risk of developing parkinsonism in the future:
The lethargy lasted from several days to a few months, and frequently culminated in coma and death secondary to respiratory failure [4]. About 80% of the patients who recovered from EL went on to develop a Parkinson’s-like disease [4, 11]. The Parkinsonian features described included tremor, bradykinesia and masked facies [12]. The disease was named post encephalitic Parkinson’s disease (PEP). Although the connection is controversial, research has shown that individuals who were born between 1888 and 1924, and thus were born or were young at approximately the time of the pandemic, had a two to three fold higher risk of developing Parkinson’s disease, than those born outside of that range [12].
Retrospective analyses of other viral outbreaks have also noted an association with increased parkinsonism following such outbreaks, including reports occurring with other influenza subtypes, hepatitis C and B viruses, Epstein-Barr virus, and Dengue virus among many others.
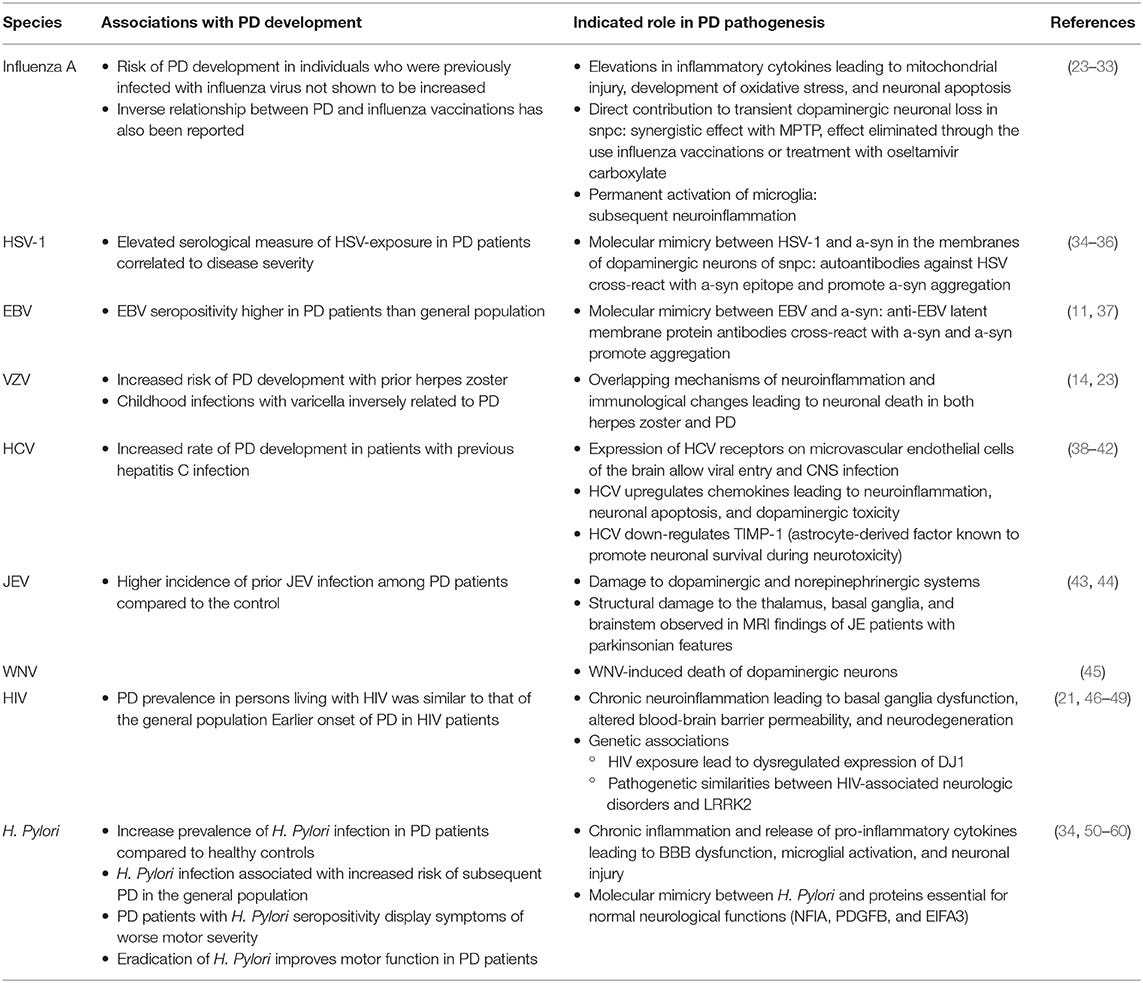
A nonexhausted list of some viruses associated with parkinsonism can be found below:
More information on viruses and their relation to parkinsonism can be found in other review articles:
Jang, et al.5: Viral Parkinsonism
Leta, et al.6: Viruses, parkinsonism and Parkinson's disease: the past, present and future.
Smeyne, et al.7: Infection and Risk of Parkinson's Disease.
Hopkins, et al.8: Viral Parkinsonism: An underdiagnosed neurological complication of Dengue virus infection.
Limphaibool, et al.9: Infectious Etiologies of Parkinsonism: Pathomechanisms and Clinical Implications.
Wouk, et al.10: Viral infections and their relationship to neurological disorders.
Wang, et al.11: Bacterial, viral, and fungal infection-related risk of Parkinson's disease: Meta-analysis of cohort and case-control studies.
The Limphaibool, et al. review also includes a table with viral agents and their pathogenesis:
Viral Pathology in Parkinson’s Disease- The Braak Hypothesis
Reasons for why viruses may lead to increased risk of parkinsonism are still unclear. Some have assumed a direct viral infection, neuronal damage, and neuroinflammation of the brain and nervous system as being a driver for the eventual neurodegeneration. Some have considered an autoimmune role, such as with Epstein-Barr virus in which anti-EBV antibodies appear to cross react with alpha-synuclein, and thus may sustain alpha-synuclein aggregation and Lewy body formation.
Interestingly, one of the first symptoms of Parkinson’s disease and some cases of parkinsonism are loss of smell as well as gastrointestinal dysfunction.
Because of this strange presentation, many hypotheses were formulated in which it was assumed that an unknown pathogen may find entry into the nervous system of individuals via passage through susceptible neurons, causing damage in the form of prion-like Lewy body aggregates which make their way to the central nervous system.
This hypothesis, so-called the Braak hypothesis12, attempted to account for these strange signs and symptoms by suggesting that specific neuronal cell types may be targeted by pathogens, thus explaining why the nose and the gut are main targets given that they may be the first places exposed to external pathogens.
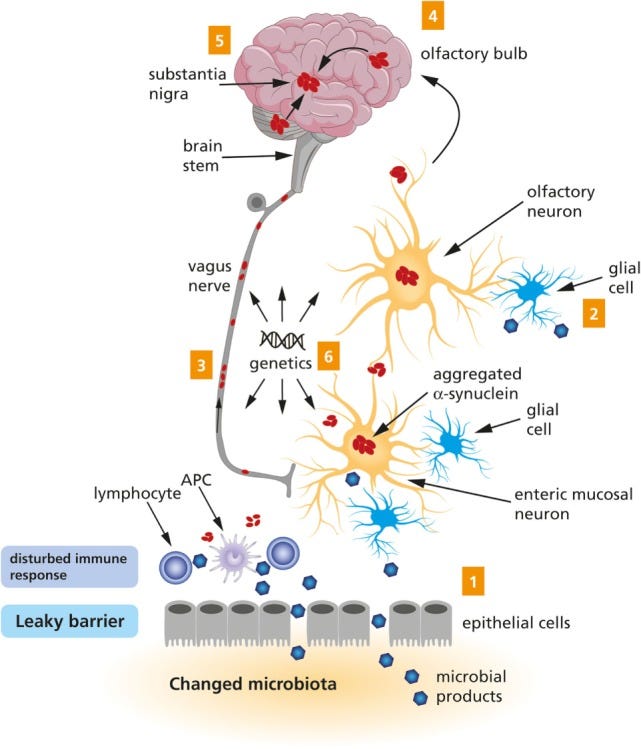
In the following years after the Braak hypothesis was proposed a “two-fold” addition was included to this hypothesis in a 2007 article from Hawkes, et al.13 with Braak as a coauthor, emphasizing that the hit in both the nose and the gut may be at play for sporadic cases of Parkinson’s disease:
We propose that an unknown neurotropic pathogen initiating the pathological process underlying sporadic PD adopts a two‐pronged attack on the nervous system: anterogradely, via olfactory pathways; and retrogradely, via enteric plexuses and preganglionic vagal fibres. If the pathogen is viral, then brain entry via the nasal route or uptake through the gastrointestinal tract has ample precedent, as described above. A nasal infection would have direct access to the olfactory nerve; infected saliva or mucous could be swallowed and reach the upper digestive tract to infect axons of Meissner's plexus and, after transneuronal passage, ascend retrogradely in preganglionic parasympathetic fibres of the vagus nerve to the lower brainstem. Direct access to the medulla via the viscerosensory fibres of the vagus in the pharynx or via the trigeminal nerve, while anatomically appealing, is not compatible with virtual sparing of the solitary tract and trigeminal nuclei during the entire course of the disorder.
There is some support for this hypothesis, as noted by Rietdijk, et al.14 [context added]:
There is experimental and clinical evidence supporting Braak’s hypothesis. Gastrointestinal problems like dysphagia, nausea, constipation and defecatory difficulty (36, 37), and the olfactory problem of the loss of smell (38) have been reported in PD. Additionally, the presence of LP [Lewy pathology] in the neurons of the olfactory tract (39, 40) and the enteric nervous system (ENS) (41–43) has been confirmed. Severe LP in the ENS [enteric nervous system] is positively correlated with constipation and motor problems in PD patients (44). There is also clinical evidence that LP in the nasal and gastrointestinal regions potentially precedes the diagnosis of the disease (32, 43, 45), leading to complaints of the digestive tract (46, 47) and problems with olfaction (48, 49) during the earlier stages of PD, before the onset of motor symptoms [this stage is also known as incidental Lewy body (LB) disease (50)].
However, not all patients diagnosed with PD exhibit loss of smell, and as noted in the previous post there’s no clear relationship between Lewy body aggregates and symptoms of PD.
It’s possible that the Braak hypothesis may account for select instances of sporadic PD, such as those that may appear after periods of viral outbreak, but it likely cannot account for all environmental causes of the diseases. Like with everything, it’s more than likely that PD and parkinsonism is a multifaceted, highly complex interplay of genetic and environmental circumstances.
Are Lewy Bodies neurotoxic or neuroprotective?
With widespread discussions of amyloid formation in relation to SARS-COV2, a question may be raised as to whether proteins related to SARS-COV2 may also interact with alpha-synuclein and cause the formation of Lew bodies.
Evidence appears to suggest that the nucleocapsid protein of SARS-COV215, as well as other SARS-COV2 proteins are able to bind to alpha-synuclein and induce Lewy body formation16.
Of course, such a mechanism may raise some concerns that the COVID pandemic may lead to increased diagnoses of parkinsonism akin to the Spanish Flu.
This topic will be explored further in the next post. However, what’s important to remember is that such studies relating amyloidogenic properties of viral proteins lack the context necessary to consider whether this behavior would be inherently dangerous or if it would actually be protective.
Last year Brian Mowrey raised this idea with a post which included an interview with Rudolph Tanzi who has done extensive research with respect to Alzheimer’s and the role of amyloid plaque formation with the disease:
Amyloid proteins, as well as alpha-synuclein, come from a class of proteins with antimicrobial properties, aptly referred to as antimicrobial peptides17.
These peptides appear to be one of the most primitive forms of innate immunity, which makes sense given their rather nondiscriminatory binding patterns, as many pathogenic proteins can bind to these peptides. And more interestingly, nearly all forms of life appear to have some type of antimicrobial peptide, from microorganisms to plants.
With such biology and evolutionary overlap for this class of proteins there would appear to be some inherent protective mechanism.
As noted in the interview with Tanzi the role of these peptides may be to adhere and aggregate around a foreign pathogen/protein. Thus, these foreign materials may serve as seeding grounds in which these protective proteins collect around to prevent cytotoxic damage.
This behavior is similar to oysters which coat irritants in a film that us humans eventually wear as pearls, as well as snow crystals in which water droplets coalesce around some material in the air to form the hexagonal constructs we see during the winter.
Therefore, this mechanism is likely done to the protection of the organism- if you can block the pathogen you’re likely to live to see another day, especially when the pathogen invades such a precarious region of the body such as the brain.
But that doesn’t mean that this event is all good.
As people get older the likelihood that one will come across various these various amyloidogenic seeds increases, and may account for the prevalence of Alzheimer’s and PD in older people.
And it’s certainly not without merit to assume that one would rather avoid any potential seeding materials if possible. It’s the balance of insult exposure and the management of insults that may determine one’s likelihood of forming neurodegenerative diseases in the future.18
But again, this raises question as to whether the COVID pandemic may result in increased diagnoses of Parkinson-related diseases in the future by serving as a trigger for neurodegeneration. More importantly, this raises questions as to the role that the COVID vaccines may play in these diseases as well, which are still yet to be elucidated.
The next post will explore viral parkinsonism as it relates to COVID, and with some discussion on the vaccine’s role if possible.
Additional Article:
Tulisiak, et al.19: Can infections trigger alpha-synucleinopathies?
Substack is my main source of income and all support helps to support me in my daily life. If you enjoyed this post and other works please consider supporting me through a paid Substack subscription or through my Ko-fi. Any bit helps, and it encourages independent creators and journalists such as myself to provide work outside of the mainstream narrative.

Vyas A, De Jesus O. Von Economo Encephalitis. [Updated 2022 Feb 16]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK567791/
Hoffman, L. A., & Vilensky, J. A. (2017). Encephalitis lethargica: 100 years after the epidemic. Brain : a journal of neurology, 140(8), 2246–2251. https://doi.org/10.1093/brain/awx177
Unlike parkinsonism relating to viral outbreaks, the emergence of encephalitis lethargica was not met with a clustering of symptoms. That is to say, there doesn’t appear to be evidence of encephalitis lethargica occurring within a household or community, with the cases appearing rather sporadic. It’s quite possible that, if caused by a virus, the manifestation of encephalitis lethargica would not be recognized in all infected individuals. However, the lack of evidence makes it difficult to rule out any specific causal entity. Some recent arguments have suggested that EL may be associated with streptococcal infections, but again that argument also lacks substantial evidentiary support. Although the outbreak of Spanish Flu in 1918 can lead to an association with EL, the reported cases of EL preceding the influenza outbreak can also argue against the viral nature of the parkinsonism given the lag.
More on this topic can be found in this review from McGall, et al.:
McCall, S., Vilensky, J. A., Gilman, S., & Taubenberger, J. K. (2008). The relationship between encephalitis lethargica and influenza: a critical analysis. Journal of neurovirology, 14(3), 177–185. https://doi.org/10.1080/13550280801995445
Henry, J., Smeyne, R. J., Jang, H., Miller, B., & Okun, M. S. (2010). Parkinsonism and neurological manifestations of influenza throughout the 20th and 21st centuries. Parkinsonism & related disorders, 16(9), 566–571. https://doi.org/10.1016/j.parkreldis.2010.06.012
Jang, H., Boltz, D. A., Webster, R. G., & Smeyne, R. J. (2009). Viral parkinsonism. Biochimica et biophysica acta, 1792(7), 714–721. https://doi.org/10.1016/j.bbadis.2008.08.001
Leta, V., Urso, D., Batzu, L., Lau, Y. H., Mathew, D., Boura, I., Raeder, V., Falup-Pecurariu, C., van Wamelen, D., & Ray Chaudhuri, K. (2022). Viruses, parkinsonism and Parkinson's disease: the past, present and future. Journal of neural transmission (Vienna, Austria : 1996), 129(9), 1119–1132. https://doi.org/10.1007/s00702-022-02536-y
Smeyne, R. J., Noyce, A. J., Byrne, M., Savica, R., & Marras, C. (2021). Infection and Risk of Parkinson's Disease. Journal of Parkinson's disease, 11(1), 31–43. https://doi.org/10.3233/JPD-202279
Hopkins, H. K., Traverse, E. M., & Barr, K. L. (2022). Viral Parkinsonism: An underdiagnosed neurological complication of Dengue virus infection. PLoS neglected tropical diseases, 16(2), e0010118. https://doi.org/10.1371/journal.pntd.0010118
Limphaibool, N., Iwanowski, P., Holstad, M. J. V., Kobylarek, D., & Kozubski, W. (2019). Infectious Etiologies of Parkinsonism: Pathomechanisms and Clinical Implications. Frontiers in neurology, 10, 652. https://doi.org/10.3389/fneur.2019.00652
Wouk, J., Rechenchoski, D. Z., Rodrigues, B. C. D., Ribelato, E. V., & Faccin-Galhardi, L. C. (2021). Viral infections and their relationship to neurological disorders. Archives of virology, 166(3), 733–753. https://doi.org/10.1007/s00705-021-04959-6
Wang, H., Liu, X., Tan, C., Zhou, W., Jiang, J., Peng, W., Zhou, X., Mo, L., & Chen, L. (2020). Bacterial, viral, and fungal infection-related risk of Parkinson's disease: Meta-analysis of cohort and case-control studies. Brain and behavior, 10(3), e01549. https://doi.org/10.1002/brb3.1549
Braak, H., Rüb, U., Gai, W. P., & Del Tredici, K. (2003). Idiopathic Parkinson's disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. Journal of neural transmission (Vienna, Austria : 1996), 110(5), 517–536. https://doi.org/10.1007/s00702-002-0808-2
Hawkes, C. H., Del Tredici, K., & Braak, H. (2007). Parkinson's disease: a dual-hit hypothesis. Neuropathology and applied neurobiology, 33(6), 599–614. https://doi.org/10.1111/j.1365-2990.2007.00874.x
Rietdijk, C. D., Perez-Pardo, P., Garssen, J., van Wezel, R. J., & Kraneveld, A. D. (2017). Exploring Braak's Hypothesis of Parkinson's Disease. Frontiers in neurology, 8, 37. https://doi.org/10.3389/fneur.2017.00037
Semerdzhiev, S. A., Fakhree, M. A. A., Segers-Nolten, I., Blum, C., & Claessens, M. M. A. E. (2022). Interactions between SARS-CoV-2 N-Protein and α-Synuclein Accelerate Amyloid Formation. ACS chemical neuroscience, 13(1), 143–150. https://doi.org/10.1021/acschemneuro.1c00666
Wu, Z., Zhang, X., Huang, Z., & Ma, K. (2022). SARS-CoV-2 Proteins Interact with Alpha Synuclein and Induce Lewy Body-like Pathology In Vitro. International journal of molecular sciences, 23(6), 3394. https://doi.org/10.3390/ijms23063394
Zhang, QY., Yan, ZB., Meng, YM. et al. Antimicrobial peptides: mechanism of action, activity and clinical potential. Military Med Res 8, 48 (2021). https://doi.org/10.1186/s40779-021-00343-2
Tanzi remarks that his two bouts of COVID have left him minorly concerned that amyloid plaque formation may have occurred. Of course, plenty of viral infections are likely to cause this formation, but again it’s more to the point that you may not want to add to the amyloid/Lewy body pile if not necessary.
Tulisiak, C. T., Mercado, G., Peelaerts, W., Brundin, L., & Brundin, P. (2019). Can infections trigger alpha-synucleinopathies?. Progress in molecular biology and translational science, 168, 299–322. https://doi.org/10.1016/bs.pmbts.2019.06.002




I have an overarching theory that I arrived at after a lot of diggning and beyond. I have a suspicion that a lot of infections by toxoplasma and similar organisms go undiagnosed, in part because the diagnostic methods are very outdated, and in part due to arrogance. and habit on the part of the medical establishment.
There have been strong connections established between this particular organism and neurological diseases, cognitive disorders, etc. etc. But the medical industry has not yet figured out how to monetize this, and so it is sitting out there as an obscure thought, and research is research, and medicine is medicine, and those two live on two separate planets.
I actually suspect that a lot of "long COVID" (perhaps not all of it but a sizeable chunk of it) is caused by undiagnosed microorganisms like toxoplasma (and possibly other ones) who have been sitting quietly or mostly quietly in many people until the immune system was disrupted, and then, once the immune system is compromised, which has happened on massive scale, incrementally and then suddenly, they rear their ugly heads, but the doctors don't even think in this direction for the most part, and so the people suffer, and keep suffering.
Anyway, the article is very detailed. If you can't access it because it is behind the paywall on Dr. Mercola's Substack, here is a link to the PDF https://media.mercola.com/ImageServer/Public/2022/September/PDF/chronic-active-toxoplasmosis-pdf.pdf
https://takecontrol.substack.com/p/chronic-active-toxoplasmosis
Thanks for this in-depth dive into parkinson's. As a physical therapist I've treated many people with this ailment, it sure is a tough one.