Consilience and additional context to yesterday's post.
Specifically, what role are eosinophils playing in these adverse reactions, and is widespread eosinophilia going undiagnosed?
This was originally intended to look through the other autopsy reports for corroborating information between the Nushida, et al.1 case report and prior autopsies. However, the look into eosinophils alone got ahead of me. Therefore, eosinophils have become the focus of this analysis.
I’ve used the term consilience quite often, and I find it fitting based on the given circumstances.
I saw it first used by Joomi Kim when describing her adverse reaction to the COVID vaccines in the following post:
Consilience refers to independent evidence that tends to converge or corroborate a specific idea or finding.
In this case, consilience can be used to describe circumstantial evidence of adverse reactions that tend to point towards a similar, hypothetical mechanism for these reactions.
Even with the limited information coming out from autopsy findings we can at least attempt to corroborate these findings with other information in order to gain some semblance of consilience.
I have thought about having an informal systematic review where I highlight all of the available autopsy reports to try to piece together some of that information, although such a feat would require a ton of time and effort. In the meantime, I’d be very appreciative if anyone has any case reports of adverse reactions available that they would not mind linking below so that I can assess and include if I find the time to do so.
With that being said, here are a few notes to add to the case report from yesterday looking at the 14-year old Japanese girl who died following a booster.
What Role are Eosinophils Playing?
Rather fascinating is the sporadic findings of eosinophils in some of the autopsy reports that have come to light.
Immunological assessment of eosinophils will be saved for another time, but a quick review will be provided.
Eosinophils are leukocytes (white blood cells) that mature from progenitor cells found in the bone marrow. They are distinguished based on the granular appearance of these cells when stained, which categorize them as granulocytes along with neutrophils and basophils. They are also sometimes referred to as acidophils due to their interaction with positively charged dyes such as eosin (hence the name).
Eosinophils can be found in various tissues and are involved with a variety of defense mechanisms, including the targeting of viruses and parasites. There are strong dynamics between eosinophils and diseases such as allergies and asthma as well. They’re also involved with many inflammatory pathways including release of cytokines and inducing inflammation, which have implicated eosinophils in various diseases of inflammation and autoimmunity. Much of this work with respect to the role eosinophils play in health have been found in recent years, although much still remains to be discovered.
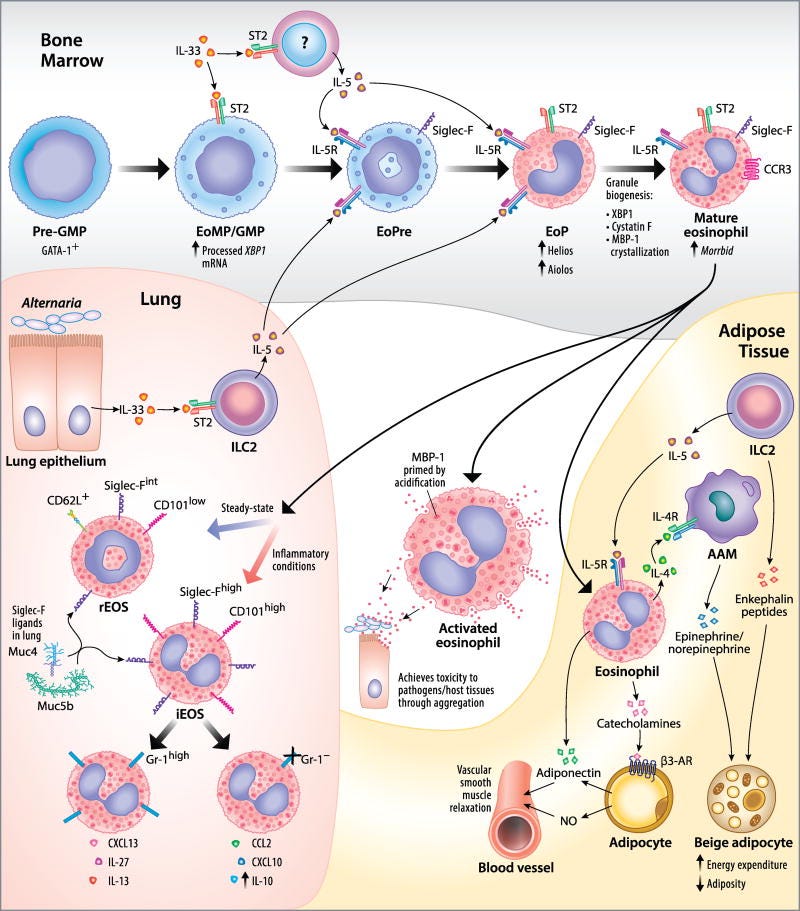
One common disease associated with eosins is called eosinophilia2, in which levels of eosinophils appear highly elevated. Tests for eosinophilia may suggest some form of inflammation, and there can be differences in tissue eosinophil level when compared to blood levels.
In the autopsy report from yesterday it was noted that the young girl had widespread eosinophil infiltration of various organs, which may suggest some form of eosinophilia (shown in the first column below).

As eosinophils are associated with inflammation it raises the question as to whether the eosinophils may have played some role in the adverse reaction experienced by this young woman and whether they are related to the multi-organ inflammation.
Not much additional information is provided with respect to the eosinophil infiltration aside from remarks about their relationship to myopericarditis.
But what’s interesting is that the pathologists in this report noted that this young girl appeared to have evidence of pneumonia, although it appears that the pneumonia was being associated with the myopericarditis as well:
Myopericarditis is a form of multiple-organ inflammation. Although pneumonia is involved, pneumonia alone is rarely a cause of sudden death, and the presence of erythrocyte-laden macrophages as well as congestive edema of the lungs on histology suggested signs of heart failure from the previous day.
What’s strange is that this assertion may have overlooked something else, in this case with respect to the role of eosinophils in this case.
In particular, there’s a unique form of pneumonia associated with eosinophils called none other than eosinophilic pneumonia.
One form of EP, acute eosinophilic pneumonia3, notes eosinophil infiltration of lungs, reduced respiration/dyspnea (shortness of breath), and lower oxygen levels (hypoxia). AEP is generally of unknown etiology, but some factors suggest that it is brought on by allergens, smoking, drugs, and may be secondary to some other event. In some cases eosinophilic pneumonia may appear similar to acute respiratory distress syndrome or may in fact be the cause of ARDS.
Here, the anecdotal account of the young girl’s sister noted that the deceased sister also had trouble breathing which woke her up in the middle of the night:
The day after vaccination, she developed a fever of 37.9 °C, which resolved by the same evening. Her sister, who had slept with her that night, reported that she woke up briefly because she was having difficulty in breathing, talked with her sister, and went to bed soon after.
Given these findings it’s interesting to consider a possible etiology of eosinophilic pneumonia, especially given the histology findings shown above.
And what’s rather fascinating is that eosinophilic pneumonia has been noted in several case reports post-COVID vaccination.
Evidence of Eosinophilia and AEP
A few examples can be seen below. I haven’t checked through each case report yet, and some of the case reports also include cases of hypereiosinophilia for reference:
Costa e Silva, et al.4: Eosinophilic Pneumonia Associated to SARS-CoV-2 Vaccine
May, et al.5: Eosinophilic pneumonia and COVID-19 vaccination
Miqdadi, A., & Herrag, M. 6: Acute Eosinophilic Pneumonia Associated With the Anti-COVID-19 Vaccine AZD1222.
Barrio Piqueras, et al.7: Acute Eosinophilic Pneumonia Following mRNA COVID-19 Vaccination: A Case Report.
Doman, et al8: Colitis with Hypereosinophilia following the Second Dose of the BNT162b2 mRNA COVID-19 Vaccine: A Case Report with a Literature Review.
Hoxha, et al.9: Hypereosinophilic Syndrome Following the BNT162b2 (BioNTech/Pfizer) Vaccine Successfully Treated with Mepolizumab: A Case Report and Review of the Literature.
Ando, et al.10: Acute asthma exacerbation due to the SARS-CoV-2 vaccine (Pfizer-BioNTech BNT162b2 messenger RNA COVID-19 vaccine [ComirnatyⓇ]).
It’s interesting that several of these case reports note people experiencing shortness of breath or difficulty breathing as the first signs of eosinophilia or AEP. I’ve personally heard of people having trouble breathing after receiving a COVID vaccine, and I’m curious if some signs of eosinophilia may be mistaken for myocarditis as it has become the main adverse reaction being examined.
In the case of the Nushida, et al. case report it appears that this adverse reaction may have been overlooked as a possible differential diagnosis, and instead was considered as being associated with the myopericarditis even though systemic infiltration of eosinophils was noted based on histology results, which also may be corroborated by the sister’s account of difficulty breathing.
Now, why this is occurring is still yet to be determined. It’s suggested that maturation of eosinophils is dictated by IL-5. The eosinophil response also appears to be associated with a Th-2 immune response.
Th-2 immune responses are generally associated with helminth infections and tissue repair, and usually is balanced with a Th-1 response. However, if left unchecked or heavily biased towards a Th-2 response the overall affect may be widespread inflammation and autoimmunity.
A quick glance noted that several of the case reports above came from those with various comorbidities, and this raises the question if predisposition towards a Th-2 response may serve as an early predictor of eosinophilia from vaccination (speculation from me based on limited information).
What’s even more interesting is that evidence of AEP has occurred during vaccine trials for the original SARS-COV vaccine. A report from 2020 even raised concerns of Th2-mediated immunopotentiation with COVID vaccines in development.
In this case, the concern detailed the possibility of increased susceptibility to eosinophilia from a SARS-COV2 infection after receiving a vaccine, which has occurred in prior animal models of SARS-COV vaccination11:

I don’t have any substantial evidence of the claims towards more severe COVID reactions, so I won’t speculate on the role of immunopotentiation for now.
But regardless, it’s interesting that there were concerns about such a phenomenon occurring prior to the vaccine rollout, which now appear to be substantiated in some regard.
Treatment for eosinophilia and AEP tend to follow a course of corticosteroids and other anti-inflammatories, which appear to be rather successful.
This again raises the question of how many severe adverse reactions or deaths may not have been accounted for, including the one reported by Nushida, et al. which confounds evidence of pneumonia as being associated with myopericarditis.
It’s curious if myocarditis has become the go-to measure of adverse reactions because it is the one most commonly talked about in mainstream press. If that’s the case, then this leads to another factor of overlooking signs and symptoms of other pathologies for one derived from a talking point. Many other adverse reactions are being detailed in the literature, but many may be overlooked for those detailing myocarditis, and in fact it is possible that many reports of adverse reactions may go unchecked because of an inherent bias to look for myocarditis first and foremost.
The treatment of corticosteroids for both myocarditis and AEP may also suggest that the pathology may resolve without ever being diagnosed and would again be overlooked.
In Regards to Consilience
The systemic eosinophilic infiltration noted by Nushida, et al. made me curious if this was age-related. However, the case reports listed above appear to be in those of various ages and predominately adults, which suggests that age may not be a factor. Rather, prior medical history and comorbidities may be better predictors of AEP and other eosinophil-related disorders post vaccination (which can be said for autoimmune diseases post-vaccination). As I haven’t looked into detail in the above case reports I won’t make any remark as of now about whether this appears to be the case.
The reports from Gill, et al. were one of the first autopsy reports to be published, and upon retrospective viewing it appears that eosinophil infiltration wasn’t as widespread as I initially reported. The pathologists note that hypersensitivity was considered as a differential diagnosis, however the lack of widespread eosinophilia raised suspicion about this diagnosis.
Rather, it appears that most of the innate immune infiltration appears to be dominated by neutrophils.
This appears to also have been the case with the study noting myocarditis in adolescents after two doses of either BioNTech or Moderna’s vaccine, which showed a skew towards elevated levels of neutrophils but lack of eosinophils (both possibly biased by outliers circled below):
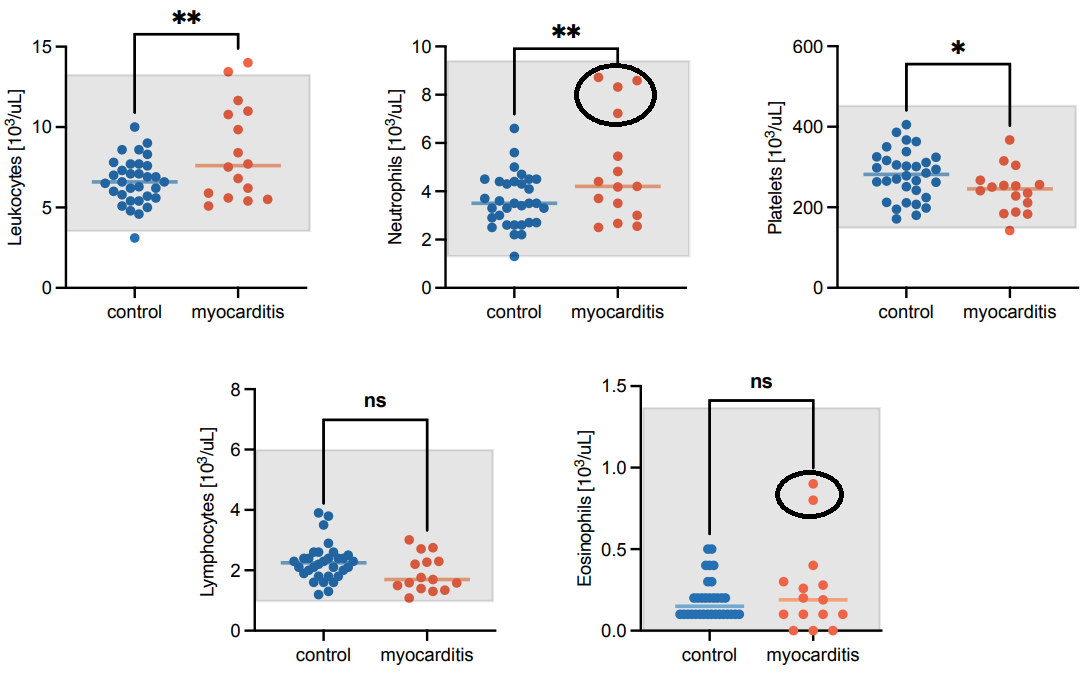
In this case it was noted that IL-5 levels were not elevated, which would corroborate the lack of eosinophils as IL-5 appears to enhance the longevity of eosinophils.
The Mörz, M. case report of the 76-year old with PD noted hypereosinophilia of cardiomyocytes as well as neuronal cell death related to hypereosinophilia. The limited data makes it difficult to assess the role of eosinophils in this case.
Overall, the presence or lack of eosinophils is rather interesting. It may be argued that the eosinophils may be related to inflammation, or they may be deployed as a compensatory mechanism for the cellular processes already underway. They may even just be bystanders and bear no relevance to the pathology. So the question still remains what exactly these eosinophils are doing at these sites.
Given the findings from Nushida, et al. it is worth raising a point that eosinophilia should be considered as a possible adverse reaction associated with COVID vaccination, with care taken to differentiate cases of myocarditis as being related to eosinophilia.
The contrast of Nushida, et al. with Gill, et al. notes that these pathologies may be distinct, especially given the widespread indication of eosinophils in the deceased young girl, the indication of pneumonia, along with the evidence of difficulty breathing as reported by her sister prior to her death. Note that I am not providing a diagnosis, but that this should be considered as a differential diagnosis that should have been investigated further.
But the question still remains why eosinophilia is occurring, why some people appear to be experiencing this adverse reaction, and whether prior medical history can provide some insights into a predisposition towards eosinophilia.
Every assessment appears to provide more questions, but that should be expected. The real question is whether evidence may come together to provide some clear indication towards consilience.
For those Interested
For those interested in learning more about eosinophils here are a few more articles on eosinophils and eosinophil-related diseases. This may be covered in more detail in another article.
Also, I promise I will cover spring-related topics soon! I tend to get too wrapped up in some of my posts and that ends up taking up more time than I would hope.
Ramirez, et al.12: Eosinophils from Physiology to Disease: A Comprehensive Review.
Wechsler, et al.13: Eosinophils in Health and Disease: A State-of-the-Art Review.
Nagata, et al.14: Mechanisms of eosinophilic inflammation.
O'Sullivan, J. A., & Bochner, B. S.15: Eosinophils and eosinophil-associated diseases: An update.
Peeples, L.16: News Feature: Avoiding pitfalls in the pursuit of a COVID-19 vaccine.
I think this article is rather revealing as it notes that the 1960s RSV vaccines were leading to a strange Th2-mediated immune response along with neutrophilia and eosinophilia, again noting that there were prior precedent for this possibility.
Substack is my main source of income and all support helps to support me in my daily life. If you enjoyed this post and other works please consider supporting me through a paid Substack subscription or through my Ko-fi. Any bit helps, and it encourages independent creators and journalists such as myself to provide work outside of the mainstream narrative.

Nushida, H., Ito, A., Kurata, H., Umemoto, H., Tokunaga, I., Iseki, H., & Nishimura, A. (2023). A case of fatal multi-organ inflammation following COVID-19 vaccination. Legal medicine (Tokyo, Japan), 63, 102244. Advance online publication. https://doi.org/10.1016/j.legalmed.2023.102244
Kanuru S, Sapra A. Eosinophilia. [Updated 2022 Sep 26]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK560929/
De Giacomi, F., Vassallo, R., Yi, E. S., & Ryu, J. H. (2018). Acute Eosinophilic Pneumonia. Causes, Diagnosis, and Management. American journal of respiratory and critical care medicine, 197(6), 728–736. https://doi.org/10.1164/rccm.201710-1967CI
Costa E Silva, M., Sá Marques, M., João, D., & Campainha, S. (2022). Eosinophilic Pneumonia Associated to SARS-CoV-2 Vaccine. Archivos de bronconeumologia, 58, 51–52. https://doi.org/10.1016/j.arbres.2021.10.008
May, J., Draper, A., & Aul, R. (2022). Eosinophilic pneumonia and COVID-19 vaccination. QJM : monthly journal of the Association of Physicians, 115(4), 251–252. https://doi.org/10.1093/qjmed/hcac041
Miqdadi, A., & Herrag, M. (2021). Acute Eosinophilic Pneumonia Associated With the Anti-COVID-19 Vaccine AZD1222. Cureus, 13(10), e18959. https://doi.org/10.7759/cureus.18959
Barrio Piqueras, M., Ezponda, A., Felgueroso, C., Urtasun, C., Di Frisco, I. M., Larrache, J. C., Bastarrika, G., & Alcaide, A. B. (2022). Acute Eosinophilic Pneumonia Following mRNA COVID-19 Vaccination: A Case Report. Archivos de bronconeumologia, 58, 53–54. https://doi.org/10.1016/j.arbres.2021.11.004
Doman, T., Saito, H., Tanaka, Y., Hirasawa, D., Endo, M., Togo, D., & Matsuda, T. (2023). Colitis with Hypereosinophilia following the Second Dose of the BNT162b2 mRNA COVID-19 Vaccine: A Case Report with a Literature Review. Internal medicine (Tokyo, Japan), 62(6), 865–869. https://doi.org/10.2169/internalmedicine.0518-22
Hoxha, A., Tomaselli, T., Minicucci, G. M., Dall'Acqua, J., Zardo, D., Simioni, P., & Naldi, L. (2023). Hypereosinophilic Syndrome Following the BNT162b2 (BioNTech/Pfizer) Vaccine Successfully Treated with Mepolizumab: A Case Report and Review of the Literature. Journal of clinical medicine, 12(6), 2376. https://doi.org/10.3390/jcm12062376
Ando, M., Satonaga, Y., Takaki, R., Yabe, M., Kan, T., Omote, E., Yamasaki, T., Komiya, K., & Hiramatsu, K. (2022). Acute asthma exacerbation due to the SARS-CoV-2 vaccine (Pfizer-BioNTech BNT162b2 messenger RNA COVID-19 vaccine [ComirnatyⓇ]). International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases, 124, 187–189. https://doi.org/10.1016/j.ijid.2022.09.019
Lindsley, A. W., Schwartz, J. T., & Rothenberg, M. E. (2020). Eosinophil responses during COVID-19 infections and coronavirus vaccination. The Journal of allergy and clinical immunology, 146(1), 1–7. https://doi.org/10.1016/j.jaci.2020.04.021
Ramirez, G. A., Yacoub, M. R., Ripa, M., Mannina, D., Cariddi, A., Saporiti, N., Ciceri, F., Castagna, A., Colombo, G., & Dagna, L. (2018). Eosinophils from Physiology to Disease: A Comprehensive Review. BioMed research international, 2018, 9095275. https://doi.org/10.1155/2018/9095275
Wechsler, M. E., Munitz, A., Ackerman, S. J., Drake, M. G., Jackson, D. J., Wardlaw, A. J., Dougan, S. K., Berdnikovs, S., Schleich, F., Matucci, A., Chanez, P., Prazma, C. M., Howarth, P., Weller, P. F., & Merkel, P. A. (2021). Eosinophils in Health and Disease: A State-of-the-Art Review. Mayo Clinic proceedings, 96(10), 2694–2707. https://doi.org/10.1016/j.mayocp.2021.04.025
Nagata, M., Nakagome, K., & Soma, T. (2020). Mechanisms of eosinophilic inflammation. Asia Pacific allergy, 10(2), e14. https://doi.org/10.5415/apallergy.2020.10.e14
O'Sullivan, J. A., & Bochner, B. S. (2018). Eosinophils and eosinophil-associated diseases: An update. The Journal of allergy and clinical immunology, 141(2), 505–517. https://doi.org/10.1016/j.jaci.2017.09.022
Peeples L. (2020). News Feature: Avoiding pitfalls in the pursuit of a COVID-19 vaccine. Proceedings of the National Academy of Sciences of the United States of America, 117(15), 8218–8221. https://doi.org/10.1073/pnas.2005456117