Are human herpesvirus reactivations being overlooked?
SARS-COV2 is not the only virus that has been associated with myocarditis, so what role do these other viruses play in severe COVID and vaccine-related adverse events?
Myocarditis and other pathologies of heart inflammation have become a hot button topic with good reason. As the COVID vaccines rolled out reports of myocarditis has raised serious concerns about the possible heart damage, both acute and lifelong, that may be occurring worldwide.
Unfortunately, all of this attention in regards to SARS-COV2 and vaccine-related myocarditis has led to these two agents in particular as being the main culprit for myocarditis and sudden cardiac deaths.
It may surprise readers that sudden cardiac death, and really viral myocarditis, has been around for many years, with a well-known virus family likely being a culprit- human herpesviruses.
The evidence has always been there since even before the pandemic that human herpesviruses, or HHV, have been associated with many forms of heart-related events, and indeed may be playing some of underlying role in the events that have transpired since the onset of the pandemic.
And again, we were made aware of this when there were frequent reports of shingles following COVID vaccination. It may be that phenotypic presentation of shingles via skin rashes made us more cognizant of this adverse reaction in particular, but underlying the shingles may have been other herpesvirus reactivations that have gone unnoticed or unchecked.
Consider that in last week’s post of a possible myocarditis “relapse” two teens presented with evidence of HHV-7 infection via PCR.
In this post I also made mention of the fact that a prior case series of vaccine-related cardiac death made mention of a possible HHV-6 infection in one of the deceased, with me at the time not really understanding the significance of this finding.
Upon first viewing all of this may seem coincidental as it’s only two case reports. However, upon digging a little deeper I believe there is more to this story, and possibly something that seemed to have been completely glossed over with a medical paradigm shift that focused on SARS-COV2 and vaccines alone.
That is, to what degree are many of these cardiac-related events related to possible HHV reactivation and infection rather than just a SARS-COV2 infection or event related to the vaccines?
There’s a lot to cover here, and I most certainly won’t get all of the details right, but we’ll explore the world of herpesviruses a bit here and go into possible connections between these family of viruses and the COVID pandemic.
Much ado about herpes
Herpesviridae are a family of DNA-based viruses. Many different forms of this viral family are known, but only a select few are known to infect humans and are designated as human herpesviruses (HHV) and given a number, likely detailing order of discovery or close associations between viruses.
Most information on HHV is clouded by the fact that two viruses in this family are known to cause the sexually-transmitted disease of genital and oral herpes- Herpes simplex, or HHV-1 and HHV-2.
Their staying power is rather obvious due to their unsightly appearance, but this has led us to forget that nearly all humans have been infected with herpesviruses of some type.
For instance, the disease we know as chicken pox is caused by a herpesvirus called Varicella zoster (HHV-3). When reactivated the virus is categorized as Herpes zoster (still designated HHV-3) and presents as shingles.
There are other viruses within this family as well, such as Epstein-Barr virus (HHV-4) which causes mononucleosis, as well as human Cytomegalovirus (HHV-5) which also causes mononucleosis as well as pneumonia.
There are other viral species as well belonging to the Roseolovirus genus, although these viruses don’t appear to have a distinct name, but rather are classified as HHV-6 and HHV-7. Herpesviruses within this genus can cause a disease known as roseola infantum1, also called exanthem subitum, which is the onset of a sudden high fever which lasts days and may lead to an appearance of rashes near the end of the viral infection.
It’s been suggested that nearly 90% of adults show evidence of HHV-6 infection, and so it’s an extremely common infection that tends to go away on its own, usually occurring by the time someone reaches the age of 2. Although common and generally mild, evidence suggests that a good portion of infants may experience febrile-related complications including febrile seizures which occur in about 15% of infants infected.
One cancer-associated herpesvirus, HHV-8, has been associated with Kaposi sarcoma (cancer that lines blood vessels and lymph nodes) and is commonly known as Kaposi sarcoma-associated herpesvirus.
Tropism and Latency
Although rather common viruses, herpesviruses tend to show rather concerning features of cell/tissue tropism as well as latency.2
Tropism refers to a bias of viruses towards certain cell types. This is usually related to the specific type of surface proteins found on viruses and which host cell receptors they interact with. For instance, SARS-COV2 is argued to show tropism for cells bearing ACEII receptors since the spike protein can bind to these receptors.
For HHV their tropism is rather widespread. For instance, many show a clear tropism for epithelia given that they are highly infectious agents. Evidence also points to their ability to infect other organs and tissues as well.
However, some show specific tropisms, such as tropism for neurons (neurotropism). HHV-1→3, in particular, show tropism for sensory ganglion and can lie dormant within these cells.
The other human HHVs show tropism for lymphocytes including B and T-cells, monocytes, macrophages, natural killer cells, and other immune-related cells.
Surprisingly (or unsurprisingly), the discovery of HHV-6 was owed in part to those with HIV, as HHV-6 was discovered within the peripheral blood mononuclear cells (PBMC) of patients with AIDS as well as those with lymphoproliferative disorders. Epidemiological surveillance would later find both HHV-6 and HHV-7 as common viruses in healthy individuals as well.
There’s probably a longstanding story between these viruses, with even some evidence suggesting that HHV-7 may compete with HIV for binding to CD4+ lymphocytes and may even disrupt HIV infections.3 I suppose COVAIDS proponents should hedge their bets given that over 85% of the population show evidence of HHV-7 infection. In fact, there’s been a rather interesting relationship between HHV and HIV given that both are considered lifelong infections, as well as due to the fact that most HIV-positive individuals are coinfected with at least one HHV.4
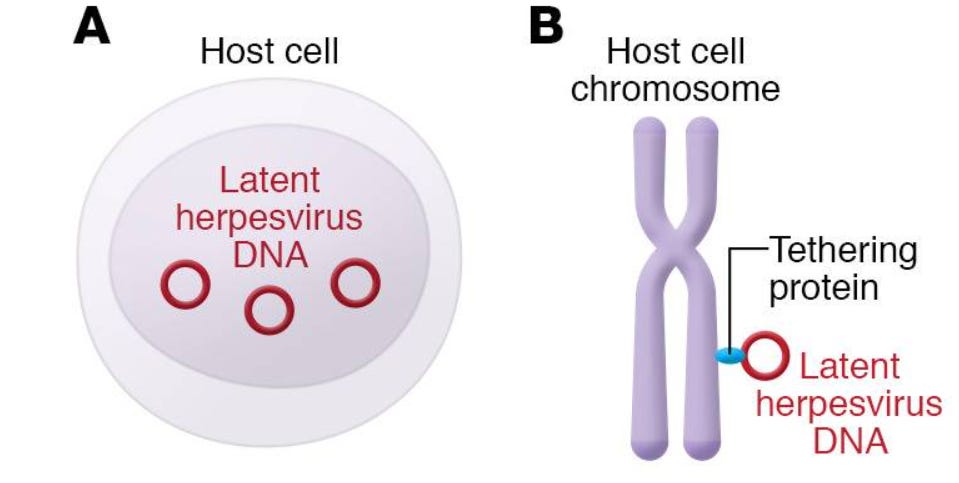
Latency refers to a time period in which a virus infects and resides within host cells without leading to cell destruction or immunological responses. Essentially, the virus is somewhat “dormant” in this stage but may become reactivated if the timing and course of host events allow for an opening, such as in immunocompromised host which may not fend off reactivated viruses.
This process is rather complex and won’t be explained in great detail. Essentially, for some of these herpesviruses their genome gets contained and circularized into a structure called an episome. These episomes can reside within an infected cell’s nucleus, or they may tether themselves to chromosomes via a tethering protein.
Again, the events in this stage is rather complex and won’t be discussed in this post.
However, what’s rather interesting is that HHV-6 is suggested to have chromosomal-integration capabilities and can integrate into the telomeres of chromosomes, and even be passed on into future generations through inheritance.
This phenomenon, called inherited chromosomally integrated human herpesvirus-6 (iciHHV-6), appears to occur in 1% of the world’s population5 and is a phenomenon in which every cell type within a person’s body contains the genome for HHV-6 due to inheritance of this integrated virus from parents (Pantry, S. N., & Medveczky, P. G.6):
Chromosomally integrated HHV-6 has been reported both in vivo and in vitro, and integration into gametes can result in the inheritance of HHV-6 [36,41,42,43]. This condition, commonly known as inherited chromosomally integrated HHV-6 (iciHHV-6), occurs in approximately 1% of the human population worldwide and is considered the major mode of congenital HHV-6 transmission [42,44]. The inherited viral genome is passed on to subsequent generations in a Mendelian manner, and all iciHHV-6-positive individuals harbor one copy of the viral genome in every nucleated cell. As a result, these individuals exhibit a persistent high viral load (1 × 106–1 × 107 copies/mL) in whole blood, and hair follicles, leukocytes, and other clinical samples are also positive [42,45,46,47,48].
There’s been some conflicting evidence as to what this integration of HHV-6 may suggest. It’s been argued that iciHHV-6 may be inactive, although some evidence suggests that some people show persistent viral replication that may be the result of reactivation of the virus or due to a superinfection with a primary infection.
The role of iciHHV-6 in health outcomes is also conflicting. Some people seem to argue that icIHHV-6 carriers may be at increased risk of cytokine overproduction, cardiovascular risk, and other possible maladies. One study noted that iciHHV-6 carriers had a much higher incident rate of angina pectoris (chest pain), which may suggest a possibly greater risk of cardiovascular complications for these individuals.7 Another study suggests that iciHHV-6 carriers have a higher antibody response, which may come from occasional gene expression of HHV-6.8 The effects of this is unknown, although it wouldn’t be surprising to consider that a persistent, heightened immune response may trigger autoimmunity or other complications since host cells are expressing these gene products.
For some context, a case report from 20219 noted an event in which a 12-year old boy presented to the hospital days after experiencing headaches, vomiting, and eventually reduced mental status. He was later found to be hypotensive, febrile, and tachycardic, and was eventually diagnosed with multisystem inflammatory syndrome in children, or MIS-C.
Eventually, further testing suggested that the boy had iciHHV-6 and may have contributed to the MIS-C in this patient.
To that, the authors made the following remarks (Biswas, et al.):
To our knowledge, an association of MIS-C with iciHHV-6 has not been previously reported. Hyperinflammatory states similar to those seen in MIS-C are known to be associated with HHV-6 activation, including in patients with iciHHV-6. In addition, proapoptotic signals activate herpesviruses and retroviruses out of latency.39,40 The end-organ dysfunction seen in MIS-C has substantial similarities to that seen in other hyperinflammatory states associated with HHV-6 activation,20,34–36 so it is plausible that in some patients with MIS-C, HHV-6 activation could contribute to the pathologies associated with MIS-C. This single case may prompt an interest in additional studies of HHV-6 in patients with MIS-C, either iciHHV-6 or activation of conventional episomally latent HHV-6. If HHV-6 is a common feature in MIS-C, that observation would constitute another contrast between MIS-C and Kawasaki disease, in which high-level HHV-6 activation has not been commonly observed.
Again, this is more about some food for thought given the reports of MIS-C related to COVID. Although the evidence tying iciHHV-6 to other diseases is weak, it still raises some questions of how latent viral infections or even integrated viruses can influence our health.
Reactivation
Herpesviruses can come out of their dormant, latent stage and reactivate. The clearest example of this being the emergence of shingles in the elderly- a sign that HHV-3 has reactivated.
The factors that contribute to reactivation are multifaceted. The most common factor is immunodeficiencies as many of these herpesviruses reactivate in severe cases of AIDS. Other factors of immune dysfunction can also contribute to this reactivation. This may be owed to reduced immune function that cannot keep latent viruses at bay, allowing them a window of opportunity to reemerge and cause havoc.
Stress is also a likely component in reactivation given that HHV-1 and HHV-2 can re-emerge as flare-ups during stressful situations. Other infections, as well as drug-induced hypersensitivity, may allow for reactivation as well. Interestingly, some findings have suggested that helminth infections may lead to HHV reactivation by altering the body’s interleukin response, which again makes room for the virus to awaken from dormancy.
The immunomodulatory properties of certain nanoparticles may also influence herpesvirus reactivation. In short, many different pathways are likely responsible for HHV reactivation.10
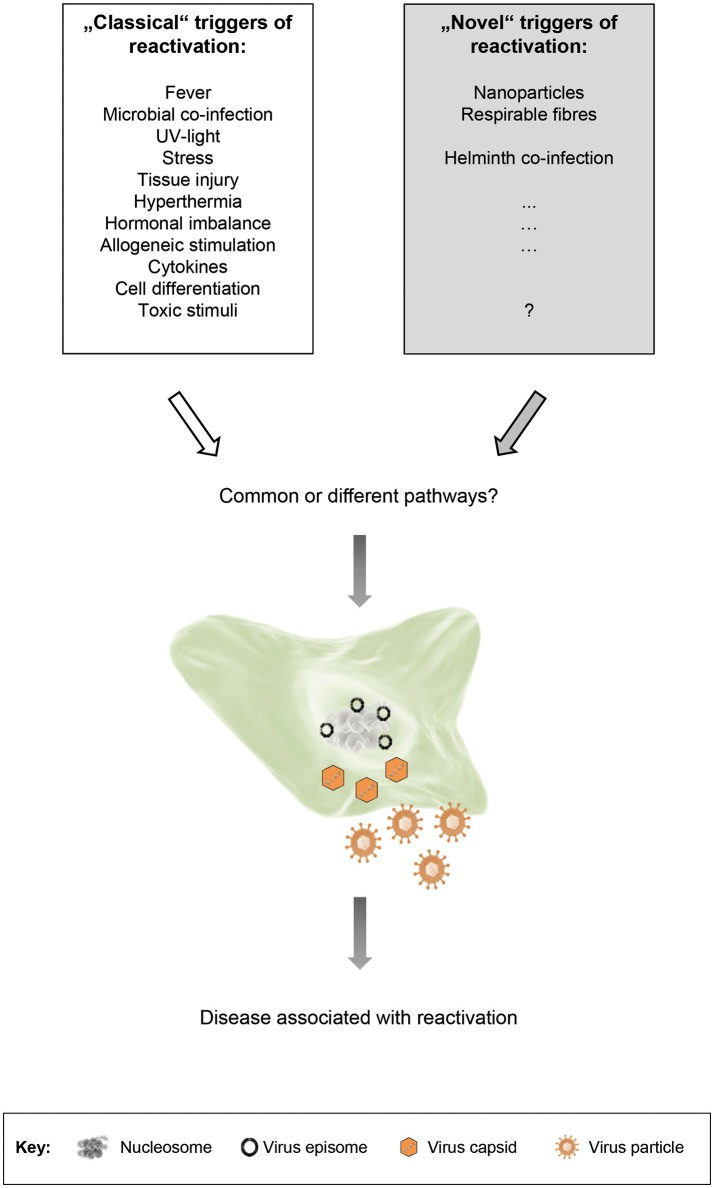
In any case, what’s important to note is that there are many different triggers for HHV reactivation. Even the use of immunosuppressants or infections with other pathogens can induce reactivation, leading to secondary infections or even worse given the lymphotropic nature of some of these HHVs, which may suggest a double-hit, synergistic effect from coinfections with HHVs.
It’s something to consider given the pandemic and what widespread infection and vaccination may mean for reactivation.
Herpesviruses, chronic fatigue syndrome, and sudden cardiac death?
Again, this article has highlighted a ubiquitous virus that readers have likely experienced at some point in their life.
And yet, these viruses appear to be associated with maladies that have gained far more attraction in recent years due to COVID, unfortunately leading to these viral agents being overlooked.
For instance, before SARS-COV2 was a “thing” there has been multiple pieces of literature implicating these viruses in post-viral syndrome and cardiovascular complications.
Several of these HHVs such as EBV has been implicated as possible drivers of chronic fatigue syndrome.11 A growing body of work is even suggesting that HHV-6 and HHV-7 may play a role in chronic fatigue syndrome as well.12,13,14
To put this into perspective, and something to consider for the next post, an article from Very Well Health published in early 2022 highlighted some long COVID haulers who were found to have reactivation of a host of HHVs, suggesting once again a possible link between the COVID pandemic, vaccines, and possible HHV reactivation. Again, food for thought for the next post.

Albeit controversial, there has also been some debate over whether HHV infection and reactivation may be associated with Alzheimer’s dementia as some of these latent viruses reside within the CNS, with some such as HHV-1→3 showing neurotropic properties in particular.15 Some of the arguments made are related to the antimicrobial nature of these amyloid plaques, and so reactivation of HHV may lead to seeding events and plaque formation as a method of dealing with viral reactivation, especially among the elderly and the immunocompromised. There’s also a possibility of neuroinflammation driving the dementia as well.16
A growing body of evidence appears to point towards HHV as being related to instances of myocarditis, heart failure and possibly sudden cardiac death, with greater attention being paid towards HHV-6 and HHV-7 in recent years. This is owed, in part, to assessments of endomyocardial biopsies for autopsy patients as well as those with idiopathic heart dysfunction where it was recognized that different viruses were present at the time of the cardiovascular incident, including HHV in some instances.
Andréoletti, et al.17 provides a bit of a summary on viral-related myocarditis:
In Europe and the USA, viral aetiologies largely prevail over other causes of myocarditis (Table 1). Coxsackie virus, parvovirus B19, HHV-6 type B and the adenovirus are the most frequent aetiological viral agents implicated in acute myocarditis in children or young adults (aged < 35 years) (Table 2) [19], [26], [27]. Human immunodeficiency virus and hepatitis C virus can be also aetiological agents of myocarditis [28], [29]. Recent data showed that it was possible to detect viruses in 67% of idiopathic left ventricular dysfunction cases using molecular techniques for the virological analysis of cardiac biopsy samples [27]. Co-infections were found in more than 12% of cases, generally HHV-6 plus parvovirus B19; HHV-6 seems to be an important cofactor in myocarditis caused by parvovirus B19 [27]. Other viruses, such as the Epstein-Barr virus or cytomegalovirus, are also associated with this pathology after heart transplantation [30].
I’ve mentioned a few reports of HHV being associated with myocarditis in the “relapse” myocarditis article including one article that Andréoletti, et al. cites above (Citation 27).18
This information may surprise some readers, but all of this suggests that SARS-COV2 wasn’t the first virus to show possible risk of heart inflammation.
There have been a few case reports of myocarditis-related severe injury and death owed to HHV infection/reactivation.
One very recent case report notes of a 40-year old woman who got ill at a family event (many other attendees were infected as well), eventually leading to complications such as myocarditis. The woman eventually needed a heart transplant.19
A 2001 case report20 notes of an 11-year old boy who died from a fatal case of myocarditis owed to a HHV-6 and Parvovirus B19 coinfection. The authors suggest that the immunosuppressive nature of HHV-6 may have exacerbated the coinfection, ultimately leading to the unfortunate death.
Reactivation of HHV has been implicated in some myocarditis deaths as well, including a Japanese man who was put on steroid therapy for a severe case of acute hepatitis, with the immunosuppressive nature of the steroids likely worsening or causing the fatal myocarditis.21 Another case report notes of two children (one 13 and one 2) who were immunosuppressed and suffered fatal myocarditis brought on by possible HHV-6 reactivation.22
So again, SARS-COV2 is not the only virus to be associated with myocarditis. In fact, it appears that we have a common family of viruses that have been associated with myocarditis and sudden cardiac death for years, with not much attention being payed towards these viruses.
However, with the onset of the pandemic a lot of this prior knowledge and information may have been overlooked in lieu of a “novel” pathogen.
Consider this case report23 of a differential, post-mortem diagnosis of fulminant myocarditis which was considered to be associated with HHV-6 rather than SARS-COV2.
The patient, like all other myocarditis patients, presented with similar symptoms:
A 59‐year‐old man was admitted to the hospital for fever and dyspnea for 3 days, resistant to therapy with antibiotic and antipyretic. His medical history showed hypertension and obesity. At admission, the clinical examination revealed: temperature of 37.7°C, heart rate of 115/min, blood pressure of 120/85 mmHg, oxygen saturation 93%, bilateral decreased breath sounds, and lower right leg reddened.
The patient tested negative for SARS-COV2 twice, and after worsening symptoms eventually died of sudden cardiac arrest.
When RT-PCR and immunofluorescence staining was conducted on myocardial samples HHV-6, rather than SARS-COV2, came up as a possible culprit:
The real‐time polymerase chain reaction (RT‐PCR) has been obtained from several frozen myocardial samples for the most common cardiotropic viral genomes, including CMV, EBV, HHV‐6, enterovirus, and SARS‐CoV‐2. All tests were negative, except for HHV‐6, which gave positive results on the myocardial sections. The RT‐PCR result has been confirmed by immunofluorescence analysis (Figure S3).
Because of the lack of autopsy reports and this focus on SARS-COV2 it’s possible that other causes of myocarditis may have been overlooked.
In that regard, the authors conclude with the following:
Macroscopic and microscopic findings demonstrated that the fatal outcome was due to an HHV‐6 related fulminant myocarditis. The patient presented clinical signs and symptoms consistent with a lot of viral infections, including COVID‐19. If it is true that during the pandemic is important to exclude SARS‐CoV‐2 infection, particularly in patients with a suspicious clinical picture, remember that clinical signs are very similar to a lot of viral infections is equally so. Myocarditis is not only caused directly or indirectly by SARS‐CoV‐2, 4 but also by a lot of cardiotropic viruses that have to rule out. 5 In other words, in patients with suggested signs of viral infection, it is crucial to get screenings for all potentially causative pathogens. In the case at hand, the clinical course of fulminant myocarditis was, as usual, rapidly progressive and in just a few hours culminating in a fatal outcome. Consequently, the cause of death and the differential diagnosis of COVID‐19 and other cardiotropic viruses were demonstrated by post‐mortem examination.
All this to say that we may have overlooked a critical component in regards to the pandemic and cases of myocarditis. It’s more than likely that these HHVs have played some sort of role within the pandemic, which either has been downplayed or overlooked.
Keep in mind that this doesn’t excuse SARS-COV2 or the vaccines. Rather, there may be something more going on, especially given the reported cases of viral reactivation post-SARS-COV2 infection as well as vaccination.
More will be explored in the next post.
Substack is my main source of income and all support helps to support me in my daily life. If you enjoyed this post and other works please consider supporting me through a paid Substack subscription or through my Ko-fi. Any bit helps, and it encourages independent creators and journalists such as myself to provide work outside of the mainstream narrative.

Mullins TB, Krishnamurthy K. Roseola Infantum. [Updated 2022 Aug 21]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK448190/
Cohen J. I. (2020). Herpesvirus latency. The Journal of clinical investigation, 130(7), 3361–3369. https://doi.org/10.1172/JCI136225
Lisco, A., Grivel, J. C., Biancotto, A., Vanpouille, C., Origgi, F., Malnati, M. S., Schols, D., Lusso, P., & Margolis, L. B. (2007). Viral interactions in human lymphoid tissue: Human herpesvirus 7 suppresses the replication of CCR5-tropic human immunodeficiency virus type 1 via CD4 modulation. Journal of virology, 81(2), 708–717. https://doi.org/10.1128/JVI.01367-06
Sara Gianella and others, The Sordid Affair Between Human Herpesvirus and HIV, The Journal of Infectious Diseases, Volume 212, Issue 6, 15 September 2015, Pages 845–852, https://doi.org/10.1093/infdis/jiv148
One rough estimates seem to suggest that this is equivalent to 40-70 million people worldwide who may have iciHHV-6, although this number I saw has to be compared to the global population at the time the article was written.
Pantry, S. N., & Medveczky, P. G. (2017). Latency, Integration, and Reactivation of Human Herpesvirus-6. Viruses, 9(7), 194. https://doi.org/10.3390/v9070194
Gravel, A., Dubuc, I., Morissette, G., Sedlak, R. H., Jerome, K. R., & Flamand, L. (2015). Inherited chromosomally integrated human herpesvirus 6 as a predisposing risk factor for the development of angina pectoris. Proceedings of the National Academy of Sciences of the United States of America, 112(26), 8058–8063. https://doi.org/10.1073/pnas.1502741112
Peddu, V., Dubuc, I., Gravel, A., Xie, H., Huang, M. L., Tenenbaum, D., Jerome, K. R., Tardif, J. C., Dubé, M. P., Flamand, L., & Greninger, A. L. (2019). Inherited Chromosomally Integrated Human Herpesvirus 6 Demonstrates Tissue-Specific RNA Expression In Vivo That Correlates with an Increased Antibody Immune Response. Journal of virology, 94(1), e01418-19. https://doi.org/10.1128/JVI.01418-19
Lisa Biswas, Noreen Crain, Michael C. Spaeder, Robert J. Gomez, Meghan Starolis, Melinda D. Poulter, Steven L. Zeichner; iciHHV-6 in a Patient With Multisystem Inflammatory Syndrome in Children. Pediatrics September 2021; 148 (3): e2021051297. 10.1542/peds.2021-051297
Stoeger, T., & Adler, H. (2019). "Novel" Triggers of Herpesvirus Reactivation and Their Potential Health Relevance. Frontiers in microbiology, 9, 3207. https://doi.org/10.3389/fmicb.2018.03207
Ariza M. E. (2021). Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: The Human Herpesviruses Are Back!. Biomolecules, 11(2), 185. https://doi.org/10.3390/biom11020185
Lee J-S, Lacerda EM, Nacul L, Kingdon CC, Norris J, O'Boyle S, Roberts Ch, Palla L, Riley EM and Cliff JM (2021) Salivary DNA Loads for Human Herpesviruses 6 and 7 Are Correlated With Disease Phenotype in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Front. Med. 8:656692. doi: 10.3389/fmed.2021.656692
Mozhgani, S. H., Rajabi, F., Qurbani, M., Erfani, Y., Yaslianifard, S., Moosavi, A., Pourrostami, K., Baradaran Bagheri, A., Soleimani, A., Behzadian, F., Safavi, M., & Rezaei, F. (2022). Human Herpesvirus 6 Infection and Risk of Chronic Fatigue Syndrome: A Systematic Review and Meta-Analysis. Intervirology, 65(1), 49–57. https://doi.org/10.1159/000517930
Philipp Schreiner, Thomas Harrer, Carmen Scheibenbogen, Stephanie Lamer, Andreas Schlosser, Robert K. Naviaux, Bhupesh K. Prusty; Human Herpesvirus-6 Reactivation, Mitochondrial Fragmentation, and the Coordination of Antiviral and Metabolic Phenotypes in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Immunohorizons 1 April 2020; 4 (4): 201–215. https://doi.org/10.4049/immunohorizons.2000006
Balin, B. J., & Hudson, A. P. (2018). Herpes viruses and Alzheimer's disease: new evidence in the debate. The Lancet. Neurology, 17(10), 839–841. https://doi.org/10.1016/S1474-4422(18)30316-8
Rizzo R. (2020). Controversial role of herpesviruses in Alzheimer's disease. PLoS pathogens, 16(6), e1008575. https://doi.org/10.1371/journal.ppat.1008575
Andréoletti, L., Lévêque, N., Boulagnon, C., Brasselet, C., & Fornes, P. (2009). Viral causes of human myocarditis. Archives of cardiovascular diseases, 102(6-7), 559–568. https://doi.org/10.1016/j.acvd.2009.04.010
Uwe Kühl, PhD, MD , Matthias Pauschinger, MD , Michel Noutsias, MD , Bettina Seeberg, MD , Thomas Bock, PhD , Dirk Lassner, PhD , Wolfgang Poller, MD , Reinhard Kandolf, PhD, MD , and Heinz-Peter Schultheiss, MD
Golob, S., Nazeer, H., Kadosh, B., Goldberg, R., Narula, N., Moazami, N., Rao, S., & Reyentovich, A. (2023). HHV-6 Myocarditis Progressing to Ventricular Standstill Requiring Cardiac Transplant. JACC. Case reports, 17, 101896. https://doi.org/10.1016/j.jaccas.2023.101896
Rohayem, J., Dinger, J., Fischer, R., Klingel, K., Kandolf, R., & Rethwilm, A. (2001). Fatal myocarditis associated with acute parvovirus B19 and human herpesvirus 6 coinfection. Journal of clinical microbiology, 39(12), 4585–4587. https://doi.org/10.1128/JCM.39.12.4585-4587.2001
Fukae, S., Ashizawa, N., Morikawa, S., & Yano, K. (2000). A fatal case of fulminant myocarditis with human herpesvirus-6 infection. Internal medicine (Tokyo, Japan), 39(8), 632–636. https://doi.org/10.2169/internalmedicine.39.632
Stefanski, H. E., Thibert, K. A., Pritchett, J., Prusty, B. K., Wagner, J. E., & Lund, T. C. (2016). Fatal Myocarditis Associated With HHV-6 Following Immunosuppression in Two Children. Pediatrics, 137(1), e20151352. https://doi.org/10.1542/peds.2015-1352
Colombo, D., Cecannecchia, C., Albore, M., Taglietti, F., Nardacci, R., Bolino, G., & Del Nonno, F. (2022). Post-mortem differential diagnosis from COVID-19: A case of fulminant myocarditis HHV-6 related. Pathology international, 72(1), 75–78. https://doi.org/10.1111/pin.13184



Impressive work...
Post shot (terrible choice) in April 2021 I felt awful. I was tested a couple of times for EBV due to mono like symptoms. There was no evidence of recent infection but my antibodies were significant. I don’t ever recall ever having mono or mono symptoms until I had the shot. This article makes perfect sense of “long covid” by reactivated herpes family viruses.