Are Catecholamines the crucial link between myocarditis and mRNA vaccines?
Part I: An overview of Catecholamines.
The cause of myocarditis among COVID vaccinees have still not been elucidated, although growing evidence continues to raise concerns about how many adverse reactions are occurring. Most notably, many concerns have been raised in regards to young, teenage boys as evidence shows this demographic is at the highest risk of developing myocarditis.
Unfortunately, the mechanisms underlying these incidences of myocarditis still continue to elude scientists- at least those who are conducting research into the matter and trying to figure out what exactly is going on.
There’s been a few hypotheses circulating, and I proposed one which associated ER Stress to heart inflammation. I haven’t revisited that hypothesis in a while, but in recent months Catecholamines have now become some of the compounds of concern, especially after an autopsy1 was conducted in two teenage boys who passed days after receiving the mRNA vaccines. The autopsy showed signs of myocarditis that differed from traditional presentations of the disease, with the conclusion suggesting that catecholamine-induced stress was the possible causative agent.
The conclusion from the autopsy was as follows:
The myocardial injury seen in these postvaccine hearts is different from typical myocarditis and has an appearance most closely resembling a catecholamine-mediated stress (toxic) cardiomyopathy. Understanding that these instances are different from typical myocarditis and that cytokine storm has a known feedback loop with catecholamines may help guide screening and therapy.
And so it was this unusual presentation that brought Catecholamines under scrutiny with respect to the mRNA vaccines, and recently a hypothesis appeared online that sheds some more light on the relationship between Catecholamines and mRNA vaccine-induced myocarditis.
It’s a very interesting hypothesis and a very approachable paper. So in this series we’ll take a look at this paper, provide some background on Catecholamines, and see what relationships we can find between these compounds and myocarditis. Afterwards, we’ll see if we can add some more context to the proposed hypothesis and expand on the idea even further.
Catecholamines Overview
Catecholamines are a class of compounds that serve both as neurotransmitters and hormones, activating many of the sympathetic nervous system pathways within our bodies and provides a regulatory mechanism for some of our bodies functions.
As the name implies, Catecholamines derive their name from the compound Catechol with an additional amine group added to the structure (hence Catecholamine).

Catechol is a benzene ring comprised of two neighboring hydroxyl (-OH) groups. Catechol itself is quite toxic and normally not found in humans. However, the addition of a carbon side chain with an additional amine group transforms the molecule into some of our post vital neurotransmitters.
The most common types of Catecholamines are Epinephrine (aka adrenaline), Norepinephrine, and Dopamine.
The structure of these 3 Catecholamines are shown below, taken from a 1972 Symposium on Catecholamines2. The important groups are indicated below:

Catecholamine Biosynthesis
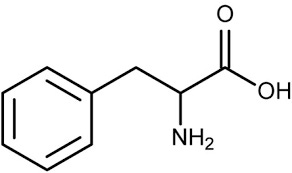
Because Catechol is not found within humans, you may wonder what is used as the starting materials to produce Catecholamines. Catecholamines are derived from the amino acid L-tyrosine with several modifications taking place through enzymatic reactions to add additional functional groups. Note that the structure of L-tyrosine already contains the backbone needed to create Catecholamines, so with a few additional fine tunes the body can produce the intended Catecholamine.

L-tyrosine can be sourced either through dietary foods or can be enzymatically converted from L-phenylalanine3 with hydroxylation adding the necessary -OH group to the para (opposite) position of the ring.
With L-tyrosine the amino acid can undergo enzymatic decarboxylation (removal of the carboxylic acid group), hydroxylation which adds an additional -OH group to different positions on the intermediate molecule, and methylation that adds an additional -CH3 group to the amine.

Regional synthesis of Catecholamines is rather interesting, as the blood generally contains circulating L-tyrosine. Serum L-tyrosine can be uptaken and converted to Dopamine in many tissues within the body.
However, biosynthesis of Epinephrine and Norepinephrine take place predominately within neuroendocrine chromaffin cells, which are located all throughout the brain as well as the adrenal gland. More specifically, the adrenal medulla is the region of the adrenal gland responsible for Epi/NE biosynthesis.

Mechanisms of Action
There are few different effects of Catecholamines, which differ based on the Catecholamine as well as the receptor being targeted.
Dopamine’s effects is mediated by dopamine receptors, and in general Dopamine is responsible for movement, memory, and reward systems within the brain.
It’s the reward system that Dopamine is well-known for, and it’s one field in which much research is being conducted in order to examine the effects of Dopamine on addiction and other psychiatric disorders (Sonne, et. al.4):
While the blood-brain barrier specifically restricts the transport of DA from the systemic circulation to the central nervous system, further research has led to the discovery of its central role in reward-seeking behavior, wherein its transmission becomes markedly increased. Current DA research includes epigenetic changes and their involvement in a variety of psychiatric conditions, including substance abuse and addiction, schizophrenia, and attention deficit disorder.[27][28] Altogether, these conditions involve disorders of the mesolimbic and mesocortical DA pathways. One common effect of addictive drugs in the CNS is the increased release of DA in the striatum, classically associated with high locomotor activity and stereotypy.[29][30] The striatal DA increase results from axon projections arising directly from the pars compacta of the substantia nigra (SN) and the ventral tegmental area (VTA), respectively, which project to the nucleus accumbens and amygdala, implicated in reward-stimulation and the fear-response.[29][31][32]
Epinephrine and Norepinephrine are responsible for the “flight-or-fight” responses that occur when our bodies experience stress (both physical and mental). Under stress5, the sympathetic nervous system responds by releasing Catecholamines which then bind to various receptors throughout the body called adrenergic receptors. Adrenergic receptors come in two categories: α and β.
Depending on which receptors Epi/NE binds to determines which physiological response occurs, including changes in heartrate, blood pressure, blood glucose levels, and heart contraction (Paravati, et. al.6):
Secretion from the adrenal medulla preceding the activation of the sympathetic nervous system functions to regulate blood pressure by contracting the smooth muscle in the vasculature (via alpha-1 receptors). The adrenergic receptors linked to blood vessels have an especially high affinity for norepinephrine relative to the other amines. Further musculoskeletal actions of catecholamines include enhanced contractility of cardiac muscle (via beta-1 receptors), contraction of the pupillary dilator (via alpha-1 receptors), piloerection (via alpha-1 receptors), and relaxation of smooth muscle in the gastrointestinal tract, urinary tract, and bronchioles (via beta-2 receptors). Both epinephrine and norepinephrine modulate metabolism to increase blood glucose levels by stimulating glycogenolysis in the liver (via beta-2 receptors), increased glucagon secretion (via beta-2 receptors), and decreased insulin secretion (via alpha-2 receptors) from the pancreas, and lipolysis in adipose tissue (via beta-3 receptors). Epinephrine also inhibits the release of mediators from mast cells and basophils in type I hypersensitivity reactions.
The effects of NE are similar to that of Epi, but as mentioned above adrenergic receptors linked to blood vessels have a greater affinity for NE meaning that higher levels of NE7 are associated with elevated vasoconstriction (narrowing of blood vessels), thus increasing blood pressure. In contrast, Epinephrine is associated with smooth muscle contraction.
These specific effects can be rather muddied, as there is a dose-dependent response between these two Catecholamines and their ability to bind to different subtypes of adrenergic receptors.
Dopamine doesn’t appear to be part of the “fight-or-flight” response, as stressors don’t appear to elevate Dopamine to the same extent as seen with Epi or NE.
Catecholamines and Disease
Given the several pathways needed for Catecholamines to elicit their effects, dysfunction among any of these pathways can lead to downstream pathophysiological alterations to health.
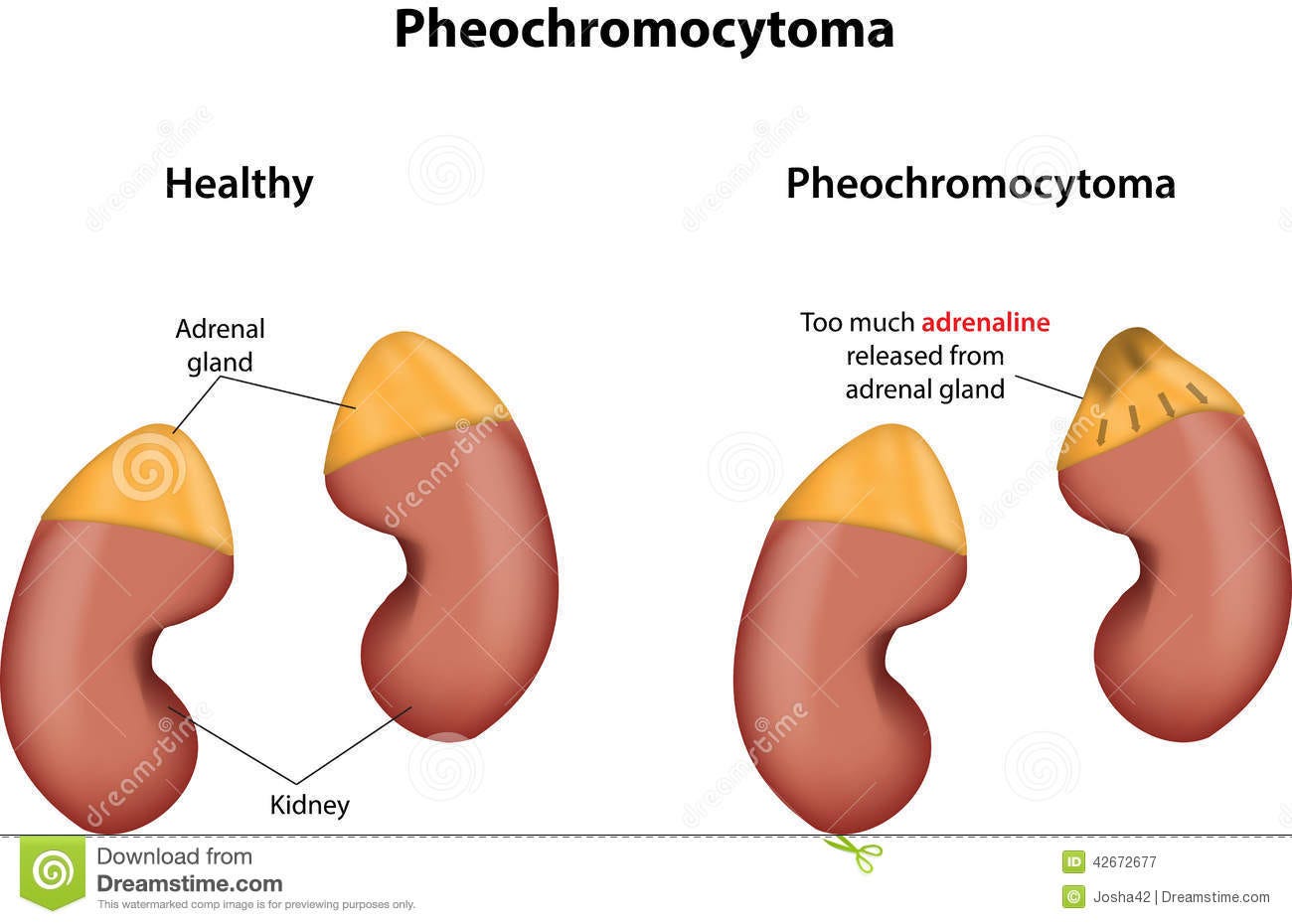
For instance, dysfunction of the adrenal medulla may alter production and release of Catecholamines. Some of these alterations may be caused by tumors called adrenal medullary tumors. Although these tumors are generally benign, these tumors may alter release of Catecholamines leading to inconsistent regulation of bodily functions such as heartrate and blood pressure, sweating, and may even lead to panic attacks. One specific example is called a pheochromocytoma, which is a tumor that develops among chromaffin cells. Tumor growth among these Catecholamine-releasing cells leads to changes in sympathetic response, and it’s usually these symptoms that may provide some context to a diagnosis of pheochromocytoma8.
Changes in expression of adrenergic and dopaminergic receptors can alter vital signaling pathways as well, and it could be through senescence and lifestyle changes that alterations in receptor expression occurs.
One fascinating aspect to Catecholamines are their high oxidative potential. Catecholamines can readily undergo oxidation within peripheral nerves and lead to the formation of quinones. It’s suspected that these quinones are “autotoxic”, in that their formation will actually harm the very same cells responsible for creating these quinones.
One other possible molecule that can be formed is the electrophilic molecule 3,4-Dihydroxyphenylacetaldehyde (DOPAL). This molecule can be formed as a side product of the enzyme monoamine oxidase9 (MOA). It’s suspected that this molecule DOPAL, as well as the quinones formed from oxidation are responsible for diseases such as Parkinson’s disease due to nerve cell death, and can form protein aggregates called Lewy Bodies which are associated with Parkinson’s disease as well as Lewy Body dementia10.

Most pertinent to this discussion is the role that Catecholamines play on the heart. In recent decades research has uncovered a disease known as takotsubo cardiomyopathy (TC), also known as broken-heart syndrome. This disease is a weakening of the heart’s left ventricle- the main chamber responsible for pumping blood- which leads to a ballooning effect. This ballooning feature gave it the name takotsubo, as the appearance resembles that of Japanese octopus pots from which the name is derived.

And the nickname for the disease is not without context, as this disease is likely to occur after sudden stressors such as natural disasters, the loss of a loved one, sudden illness, or sudden physical or mental stressors.
Originally considered benign, recent evidence has suggested that takotsubo cardiomyopathy is far more dangerous and can be deadly. Diagnoses of this form of cardiomyopathy has increased, likely due to improved diagnostic techniques or due to accrual of every day stressors caused by modernity.
Symptoms of TC overlap with those of heart attacks including chest pain, shortness of press, and abnormal presentations on EKGs. Imaging is likely also to reveal the ballooning of the left ventricle as seen in the image above.
TC generally presents in older women post-menopause, although recent evidence suggests that younger patients who present with this disease are likely to be male- sound familiar?
The cause of TC has not been fully elucidated, but some of the evidence points towards Catecholamines being a driving force. Post-menopausal women tend to show elevated sympathetic nervous system responses, and stressors are likely to engage the sympathetic nervous system as well. Evidence also points towards elevated Catecholamine levels during a presentation of TC (Amin, et. al.11):
A study by Wittstein et al. revealed that the plasma levels of epinephrine were critically elevated in TC patients, with emotional stress as its major precipitating factor. In addition, the study also indicated that the serum catecholamine concentration was two to three folds higher in TC than MI patients [19]. Moreover, other studies also substantiate the catecholamine theory further through exogenously administered catecholamine and pheochromocytoma, resulting in similar features of TC [20-21]. Excessive levels of catecholamines released by the sympathetic nervous system caused by a stressful condition could result in intracellular calcium overload and cardiac dysfunction through b(1)- adrenoreceptor signal transduction pathway (Figure 1) [4].
And this makes sense, as Catecholamines are responsible for the “flight-or-fight” response and under stressful situations Catecholamines may be released that alter the proper functioning of the heart and blood flow. Repeat stressors are likely to then elevate this dysfunction leading to alterations to the left ventricles and possible ballooning.
This evidence doesn’t intrinsically tie into the issues of myocarditis, but it does provide for background knowledge that supports the idea of Catecholamines playing a role in heart disease. As such, there is a basis to argue that myocarditis may be related to Catecholamine release as supported by the autopsy report of the two adolescent males.
As always, this post is not intended to be exhaustive, but it provides a look into Catecholamines. This provides for some background knowledge that can aid in assessing the hypothesis put forth by Dr. Cadegiani. We’ll take a look at his hypothesis next.
If you enjoyed this post and other works please consider supporting me through a paid Substack subscription or through my Ko-fi. Any bit helps, and it encourages independent creators and journalists outside the mainstream.

James R. Gill, Randy Tashjian, Emily Duncanson; Autopsy Histopathologic Cardiac Findings in 2 Adolescents Following the Second COVID-19 Vaccine Dose. Arch Pathol Lab Med 1 August 2022; 146 (8): 925–929. doi: https://doi.org/10.5858/arpa.2021-0435-SA
Catecholamines--a symposium. (1972). California medicine, 117(3), 32–62.
Sonne J, Goyal A, Lopez-Ojeda W. Dopamine. [Updated 2022 Jul 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK535451/
Note that stress here is not being used as a synonym for distress. Stress is anything that puts mental or physiological pressure onto the body. This includes environmental changes such as stark changes in temperature or weather. Keep this in mind as explanations of stress and the sympathetic nervous system take the “flight-or-fight” explanation a bit too literally.
Paravati S, Rosani A, Warrington SJ. Physiology, Catecholamines. [Updated 2021 Oct 30]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507716/
Exogenous Norepinephrine is used as a way of treating hypotension.
The MayoClinic provides a few symptoms below:
Oxidation of the amine generally leads to the formation of a carboxylic acid. In some regards, this oxidation by MOA may be considered incomplete resulting in the electrophilic catecholaldehyde DOPAL.
Goldstein D. S. (2021). The Catecholaldehyde Hypothesis for the Pathogenesis of Catecholaminergic Neurodegeneration: What We Know and What We Do Not Know. International journal of molecular sciences, 22(11), 5999. https://doi.org/10.3390/ijms22115999
Amin, H. Z., Amin, L. Z., & Pradipta, A. (2020). Takotsubo Cardiomyopathy: A Brief Review. Journal of medicine and life, 13(1), 3–7. https://doi.org/10.25122/jml-2018-0067
Additional information in regards to treatment and presentation of TC can be seen from this review:
Singh, T., Khan, H., Gamble, D. T., Scally, C., Newby, D. E., & Dawson, D. (2022). Takotsubo Syndrome: Pathophysiology, Emerging Concepts, and Clinical Implications. Circulation, 145(13), 1002–1019. https://doi.org/10.1161/CIRCULATIONAHA.121.055854







Magnesium inadequacy is likely due to spike effects on the ankyrin binding domain of TRP channels. Topical magnesium sources or chelated magnesium supplements are probably needed.
"These findings probably reflect consequences of high intracellular concentrations of Ca2+, and it has been proposed that Ca2+ overload in myocardial cells produces the ventricular dysfunction in catecholamine cardiotoxicity.20 "
Takotsubo Cardiomyopathy - A New Form of Acute, Reversible Heart Failure https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.108.767012
Thanks. Looking forward to the next installment!