Note: Some figures have been removed to fit email size limit. Please refer to the website for additional figures.
Even a year after these vaccine rollouts many people- including myself- have remained unvaccinated and have been quite hesitant of these mRNA vaccines built on brand new mechanisms and processes.
For those who may have held out, they may have been in search of a more traditional vaccine that either utilizes an attenuated virus or focuses solely on the presentation of the necessary antigen. Novavax has promised to fill this empty spot in the market with their antigen-based vaccine.
I know many of my readers have questions about whether Novavax would be a better alternative, so I a decided to provide a brief look and see if it may be a safer vaccine option.
Novavax’s Vaccine Overview
Novavax’s vaccine, named Nuvaxovid or Covavax (NVX-CoV2373) follows a more traditional vaccine design with a few bells and whistles added. This vaccine is considered a protein/antigen-based vaccine, as it utilizes the actually spike protein of the SARS-COV2 virus rather than utilizing mRNA technology to produce the spike.
While mRNA vaccines are the “Blue Aprons” of the vaccine world- order the vaccine and assemble it yourself- Novavax’s vaccine is the takeout version- order it, pick it up, and you’re done. No assembly needed.
But the spike protein can’t quite exist on its own. It needs some help in its presentation. Here, Novavax utilizes an interesting technology called an Immune Stimulating COMplex, or ISCOM for short. ISCOMs are comprised of a compound that is intended to stimulate an immune response (called an adjuvant) as well as a good mix of cholesterol and phospholipids. It essentially mimics a cell membrane studded with the antigen and serves as a way to present the antigen to the immune system. For Novavax, their ISCOM is a propriety formulation called Matrix-M and contains all of the agents indicated above.
The overall ISCOM structure supposedly looks similar to that of an antigen-studded tennis ball:

This structure is quite unique, as it doesn’t have to present with only one antigen. Instead, this ISCOM structure can present with several antigens including those from other respiratory viruses. Just a few days ago Novavax reported the initial results of a Phase I/II clinical trial of a vaccine utilizing both SARS-COV2 and Influenza antigens. This “antigen cocktail” is intended to provide several vaccines in one go rather than the multiple typically needed.
The immune-stimulating adjuvant that makes up this ISCOM is a saponin called Quillaja saponins and are derived from the Quillaja saponaria Molina plant found in South American countries such as Peru and Chile.

Saponins derive their name from the latin root sapon which means soap1. When added to water saponins form bubbles. This comes from the amphiphilic nature of saponins which are comprised of polar, water-loving groups as well as nonpolar, lipid-loving groups. This allows them to serve as good emulsifiers by interacting with both water and lipid-based structures, allowing for the formation of the ISCOM structure while aiding in the structure’s overall solubility. Because of this, Q. saponins have been used extensively in many foods and cosmetics (from Fleck et. al.2):
Saponins from Q. saponaria have a wide range of applications. Historically, Quillaja bark saponins have been used as a detergent. Nowadays they are approved for use as food additives in 187 signatory countries of the Codex Alimentarius, including the European Union, the United Kingdom, the United States, China, and Japan [6], the latter of which also allows its use in cosmetics [7]. The European Commission of Cosmetic Ingredients (CosIng) database has listed some Quillaja products as cosmetic ingredients, such as the bark, bark extract, root extract, and wood extract. Depending on the material used, different functions are attributed, such as antidandruff, cleansing, emulsifying, foaming, masking, moisturizing, skin conditioning, and surfactant [8].
Nowadays, there is increasing interest in using saponins as natural emulsifiers, alone or blended with others. Numerous studies have reported that Quillaja saponins are good emulsifiers for O/W emulsions [7,9,10] and nanoemulsions [10]. Recently, a food ingredient containing Quillaja bark saponins (Q-Naturale®) was approved by the FDA as an effective emulsifier for beverages [6,10].
These Q. saponins, as adjuvants, are intended to stimulate the innate and adaptive immune response, and helps to recruit many of the immune cells necessary for targeting the antigens3. So not only does Novavax’s COVID vaccine present the antigen, but it also stimulates the immune system and provides a more robust immune response.
That leaves us with the spike protein. The spike protein used in Novavax’s vaccine is the full-length spike glycoprotein...of the Wuhan strain (from Dunkle et. al.):
NVX-CoV2373 (Novavax), a SARS-CoV-2 vaccine made up of full-length, stabilized, prefusion, recombinant spike protein trimers produced from the Wuhan-Hu-1 sequence, is assembled into nanoparticles coformulated with a saponin-based adjuvant (Matrix-M).
So this vaccine is likely to suffer from the same issues that the Adenovirus and mRNA vaccines suffer from as they are presenting an old antigen that is no longer circulating among the population4. Keep in mind that we don’t have any data of Nuvaxovid against Omicron aside from evidence about a booster’s effectiveness.
This brings us to another caveat of Nuvaxovid. It is scheduled to be a two-dose regimen separated out by 21 days rather than 28. Why is this vaccine a two-dose regimen just like the mRNA ones, I am not sure. I am not quite sure why the mRNA ones were decided to be 2-dose, but just keep in mind that if this is a vaccine worth considering that it will likely be a 2-dose regimen (3-dose to apparently deal with Omicron…).
Lastly, each dose of Nuvaxovid is 0.5mL containing 5ug of recombinant spike and 50ug of Matrix-MTM administered intramuscularly.
Clinical Trials, or Groundhog Day?
So, I hate to break some bad news for many of you. This post is intended to examine whether Novavax’s vaccine is safer and effective relative to the mRNA vaccines. Unfortunately, the clinical trials here suffer from all of the same issues as the clinical trials for the mRNA and Adenovirus vaccines.
This includes a low number of participants (which may mask adverse reactions that require larger samples sizes) as well as the measure of “relative vaccine effectiveness” as measured by rt-PCR or hospitalizations and death. Considering that, in nearly all clinical trials, the rate of positive COVID tests are low in both the treatment group and the placebo group the overall absolute risk5 would still be considered to be low.
One strength of these Nuvaxovid clinical trials is that they occurred during early 2021 and thus were conducted at the time that Alpha was the most dominate variant (the most recent clinical trial also included a bit of other variants such as Beta and Gamma as well) and at least provides some consideration against variants. But again, Omicron is far removed from prior variants which were far more related, and therefore this evidence may not be comparable to the current variant.
For those who would like to examine these trials in further detail here are the following studies:
Phase 1–2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine (Keech et. al.)
The results of this trial may be considered minimal, especially in light of the other clinical trials. The purpose of these trials are to see whether acute toxicity and reactogenicity may occur from these vaccines. Matrix-M have been used for quite some time, and the only possible agent in question, the saponin, is used extensively in many products. There is a matter of the spike protein, but considering that these spike proteins are supposed to be similar to the ones in the mRNA vaccines we are not likely to notice anything from such a small trial. The only possible fruitful result of these studies are to measure a dosage that provides the best level of immunogenicity.
For example, here is the study design:
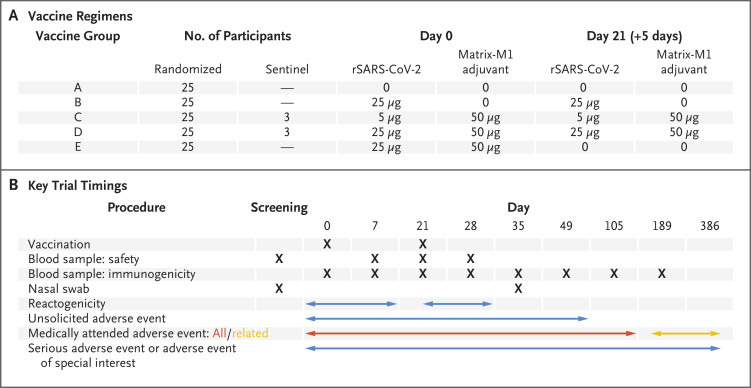
In these studies both 5ug and 25ug of recombinant spike were assessed. Of course, we know that the dosage that Novavax went with is the 5ug dose. This is likely due to to the similar immune response to both doses mostly aided by the adjuvant.

As for safety, the measures were fairly broad and measured aspects such as headaches, myalgia, fever, and nausea, but no myocarditis or pericarditis, as well as neurological impairments (myocarditis and pericarditis are mentioned in the supplemental material, but don’t appear to have been evaluated in this clinical trial). The most severe systemic events were joint pain and fatigue and was seen in both treatment and placebo groups.
The researchers state in the discussion:
The primary safety and immunogenicity analyses indicate that in healthy adult participants 18 to 59 years of age, two-dose regimens of 5 μg and 25 μg of rSARS-CoV-2 plus the Matrix-M1 adjuvant had acceptable safety findings and induced high immune responses, with levels of neutralizing antibodies that closely correlated with anti-spike IgG. Furthermore, neutralizing antibody responses after the second vaccination with rSARS-CoV-2 plus Matrix-M1 exceeded values seen in symptomatic Covid-19 outpatients and were of the magnitude seen in convalescent serum from hospitalized patients with Covid-19. The benefit of the Matrix-M1 adjuvant was clear in the magnitude of the antibody and the T-cell response, the induction of functional antibodies, and antigen dose sparing. The value of the second dose on day 21 for the two-dose rSARS-CoV-2 plus Matrix-M1 regimen is clearly demonstrated and warrants the use of this vaccination schedule.
Safety and Efficacy of NVX-CoV2373 Covid-19 Vaccine (Heath et. al.)
&
Efficacy and Safety of NVX-CoV2373 in Adults in the United States and Mexico (Dunkle et. al.)
Remember that these results suffer from all of the issues that prior clinical trials with these COVID vaccines suffer from. Keep that in mind when examining the evidence.
Both of these clinical trials are Phase III trials. The former was conducted in the UK while the latter was conducted at sites within both the US and Mexico.
In both cases the 5ug recombinant spike dose was used along with 50ug Matrix M. Primary endpoints included positive rt-PCR tests 7 days post-2nd dose as well as hospitalization and deaths.
The vaccine efficacy against mild COVID was 89.7% in the UK study while the US/Mexico study had a vaccine efficacy of 90.4% based on rt-PCR positivity rates. This value, in light of the results of the prior vaccines, may not mean much. What’s important would be whether these vaccines elicited the proper immune response in the nose and pharynx. Unfortunately, these studies are not powered to measure such immune responses.
Within the UK trial 5 instances of severe COVID were found, all within the placebo group- although no deaths took place. For the US/Mexico trial, 14 placebo participants suffered from moderate-severe COVID (10 moderate, 4 severe). This was considered to be a 100% vaccine efficacy against severe COVID in both trials. Again, whether these results amount to anything is a different matter. Personally, I think we can see the problems with such measurements.
What’s interesting is that there was one instance of myocarditis recorded in the UK trial. The researchers suggest this could have come from a viral infection. Whether this is true or not, it’s interesting that this was not verified by the researchers:
One related serious adverse event (myocarditis) was reported in a vaccine recipient, which occurred 3 days after the second dose and was considered to be a potentially immune-mediated condition; an independent safety monitoring committee considered the event most likely to be viral myocarditis. The participant had a full recovery after 2 days of hospitalization. No episodes of anaphylaxis or vaccine-associated enhanced Covid-19 were reported.
There were also reports of myocarditis in the US/Mexico trial, although it occurred in both the vaccine and placebo group (the wording by the researchers to there being “no imbalance in myocarditis and pericarditis” is confusing, but the rates appeared to be the same).
From this data alone it is hard to assess the safety of Novavax’s vaccine compared to those of the mRNA vaccines.
The discussion from the UK trial indicates the following:
A two-dose regimen of the NVX-CoV2373 vaccine administered 21 days apart was found to be safe and 89.7% effective against symptomatic Covid-19 caused by both B.1.1.7 and non-B.1.1.7 variants. The timing of accumulated cases in this trial allowed for a post hoc assessment of vaccine efficacy against strains that included the B.1.1.7 variant, which is now circulating widely outside the United Kingdom and was the most widespread strain reported in the United States at the time of this report15 (Table S5). This variant is known to be more transmissible and to be associated with a higher case fatality rate than previous strains.7 Vaccine efficacy was greater than that associated with the ChAdOx1 nCoV-19 vaccine (AstraZeneca) (70.4%) in a smaller phase 2–3 trial.16
And this is from the US/Mexico trial:
In this randomized, controlled trial assessing the efficacy and safety of an adjuvanted SARS-CoV-2 recombinant spike protein vaccine in approximately 30,000 participants, we continue to assess, in a blinded fashion, the duration of protection after blinded crossover, as well as the overall safety profile in the trial participants. The trial includes a demographically diverse population in the United States and Mexico and provides strong evidence of high short-term vaccine efficacy of NVX-CoV2373 for the prevention of Covid-19 (>90%) and for the prevention of moderate-to-severe disease (100%).
Different Vaccine; Same Issues
On the surface, Novavax’s Nuvaxovid vaccine may seem promising based on the Phase III clinical trial data. If it weren’t for the evidence of prior vaccines, these results may have seemed very convincing.
But we aren’t in the beginning stages of the vaccine rollout. We are nearly a year in, and we should be a year wiser, meaning we should have disabused ourselves of the many issues that we now notice from these prior clinical trials.
They are not powered to measure adverse reactions in such a small sample size, and thus we can’t look at these Nuvaxovid results as a validation for these vaccines safety. When it comes to efficacy, we can’t quite tell because of the same issues as these mRNA and Adenovirus vaccines. It is highly unlikely that Nuvaxovid will be a neutralizing vaccine as they are more aligned with the antigen presentation seen in typical Influenza vaccines, which already have a fairly low efficacy rate.
Unfortunately, for Nuvaxovid we may not realize if there are any safety concerns until the vaccine is provided to millions of people, and at that point we may run into the same issues with the other vaccines.
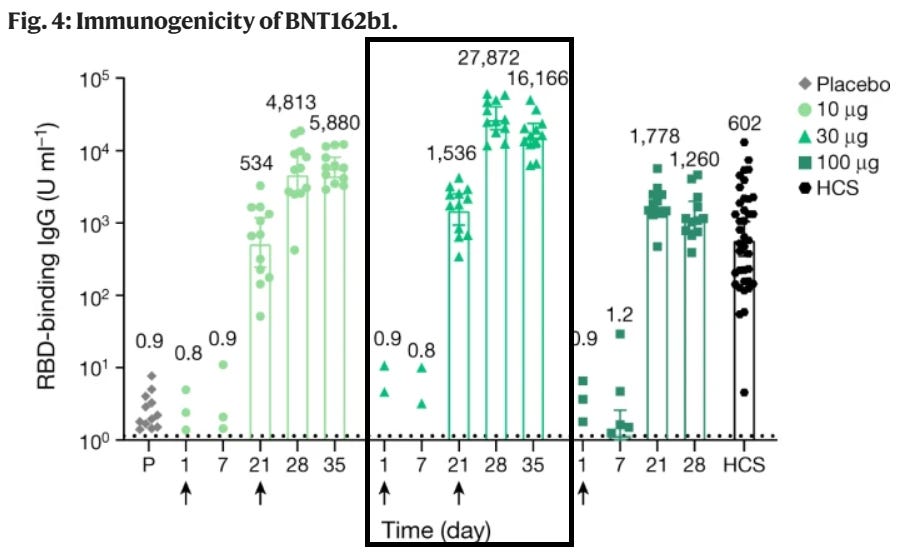
A promising aspect for Nuvaxovid may be the adjuvant nature of the saponins. Compared to Pfizer’s Phase I/II clinical trials6, Nuvaxovid appears to elicit a stronger anti-Spike IgG response, although Pfizer’s trial looked specifically at RBD-binding antibodies while Novavax’s looked at anti-spike IgG overall. That may be one advantage to Nuvaxovid, but again we will not know the real-world effects until this is provided to millions of people, and I would argue that many people may not want to be considered as possible guinea pigs in other vaccine trials7.
And there is another distinction to make with Nuvaxovid. Because these vaccines are antigen-based, it may help us to answer one question- is it the process of spike production, requiring a person’s cellular machinery and possible ER stress and production of pro-inflammatory markers that may be causing the many adverse reactions we are seeing, or is it the spike protein alone? If we do see many of the same issues with Nuvaxovid, we may be able to conclude that it is the spike protein that serves as the main culprit- a finding that may require a large deployment of Nuvaxovid, and whether this is something worth risking has been a huge concern for many of us.
These vaccines have put us in a precarious situation, one where the cure may be the disease. But it’s also at a point where many of the medical establishments that we have relied on for decades have taken a position of hubris, relying more on their positions of authority more than the evidence sought out through scientific inquiry. They have acted from a position of ignorance- not knowing the actual long-term effects of these vaccines- while also coming from a place of arrogance- espousing the safety of these vaccines to which we have no longterm safety data for.
Overall, it’s hard to determine whether Novavax’s Nuvaxovid fares any better in safety to the vaccines we have on the market, and for those looking for an alternative this may be a bit disheartening (note- I am not a doctor and this should not be used as medical advice!).
It’s also quite strange that the FDA has “slow-walking” the EUA approval for Nuvaxovid. So far there is no estimated EUA date, leading to questions as to why the review process is taking so long for this specific vaccine. Investors are wondering as well, and so far this is leading to Novavax’s stock value to continuously decline.
Maybe there is something behind the scenes going on; maybe there is a vested interest in the mRNA vaccines alone. Either way, we will still need more evidence, more data, and less arrogance from those in authority.
Quillaja saponins are technically considered detergents and not soaps, although they are very similar in nature. The usually difference comes from the polar head groups as soaps tend to be comprised of carbonates and a soft cation such as sodium while detergents may usually utilize a sulfate head group and harder cations such as calcium or magnesium ions. The Quillaja saponin structure is unique and it is actually considered a detergent, meaning that the naming may be a bit more ambiguous rather than discrete.
Fleck, J. D., Betti, A. H., da Silva, F. P., Troian, E. A., Olivaro, C., Ferreira, F., & Verza, S. G. (2019). Saponins from Quillaja saponaria and Quillaja brasiliensis: Particular Chemical Characteristics and Biological Activities. Molecules (Basel, Switzerland), 24(1), 171. https://doi.org/10.3390/molecules24010171
Reimer, J. M., Karlsson, K. H., Lövgren-Bengtsson, K., Magnusson, S. E., Fuentes, A., & Stertman, L. (2012). Matrix-M™ adjuvant induces local recruitment, activation and maturation of central immune cells in absence of antigen. PloS one, 7(7), e41451. https://doi.org/10.1371/journal.pone.0041451
Unlike the mRNA vaccine, the fix for a new antigen is not as easy for Novavax. The mRNA vaccines only need to theoretically use the sequence that codes for a different variant, such as the spike protein for Omicron. However, Novavax being a protein/antigen vaccine would need to manufacture and mass produce this new antigen. This would require either viral production or recombination with yeast or bacteria to produce the spike. Under both scenarios the spike would have to be isolated and purified from the production source. Overall, the process is much more difficult and time-consuming, and likely explains why this vaccine is coming so much later than the mRNA-based vaccines.
To clarify on relative risk vs absolute risk, you may have heard the seatbelt analogy used. The seatbelt analogy examines the severity of an illness or death for someone who wears a seatbelt in a car crash vs someone who didn’t wear a seatbelt in a car accident. We may find evidence that seatbelts tend to reduce severity of harm and death, but there’s one thing that is missed- how likely are people to get into accidents in general? The incidences of car accidents are absolute risk reductions. They take into account not just when the scenario or incident happens, but how often they occur. With these COVID vaccines, the relative risk reduction argues that in the incident that someone comes into contact with someone else with COVID they are less likely to become infected or symptomatic. However, absolute risk would examine how likely someone is to come into contact with someone who is infected, which was relatively low before Omicron. Absolute risk supercedes relative risk, as it measures the likelihood of the incident to occur in the first place.
What’s interesting is that several participants were unblinded during the US/Mexico vaccine trial because the mRNA vaccines were beginning to be distributed, meaning that the results of this study may have been slightly affected by these circumstances. The researchers noted that participants used local (injection site) reactions as a measure of whether they were given a placebo or the vaccine, and based on their own opinion decided to unblind themselves.




I would think that not using your own cells as antigen factories has to be a plus when it comes to safety. If I had to take one, I suppose it would be this one, except that:
- I don't have to
- It's not available here in the USA
- The omicron spike is so different from the Wuhan spike that antibodies created by any of the existing vaccines are effectively useless now.
Thanks for all your work. I super appreciate it. I have had covid twice, once before vaccines and then omicron. I would not want to disturb this immunity with any kind of vaccine. And since many many more people have had covid than registered, it is certainly not a good idea to get vaccinated unless you know if you had it or not: or personally period. I imagine you know all this but I thought I would put in a quote from Time about the CDC study to determine how many people have had covid: "In the CDC’s new study, researchers used data on antibodies—proteins the body generates to fight off an infection—to better understand how many people in the U.S. previously had COVID-19. Antibodies made to fight off SARS-CoV-2 (the virus that causes COVID-19) are distinct from those produced by vaccines, so testing for these proteins can help determine if someone was previously infected, even unknowingly." Whoops I guess they slipped when they admitted that the antibodies from the disease are different than the vaccines.