A Look at Evusheld
An overview into AstraZeneca's prophylactic monoclonal antibody treatment for the prevention of COVID.
A few weeks ago reader Heidi Heil alerted me to a new type of monoclonal antibody that is one of the only prophylactic treatments called Evusheld. With everything going on I thought it may be fitting to provide a more general overview to inform readers about possible therapeutic options out there.
Although this post will focus solely on Evusheld, please check out previous works on Monoclonals in my Anthology Series for additional information, which may help supplement this post. Just note that much of the information may be outdated due to the emergence of Ba.4/Ba.5.
Also, please note that the information here should be intended to be informative, not prescriptive. Please use this information to inform yourself and consult with medical professionals and a physicians when considering treatment.
General Overview
Evusheld (AZD7442) is a combination monoclonal antibody therapeutic from AstraZeneca designed in partnership with Vanderbilt University Medical Center. Evusheld is comprised of two monoclonals; Cilgavimab (AZD1061) and Tixagevimab (AZD8895). Both antibodies were derived from memory B cells from people previously infected with SARS-COV2.
Evusheld, formerly known as AZD7442, is a combination of two long-acting antibodies - tixagevimab (AZD8895) and cilgavimab (AZD1061) - derived from B-cells donated by individuals previously infected with the SARS-CoV-2 virus. Discovered by Vanderbilt University Medical Center and licensed to AstraZeneca in June 2020, the human monoclonal antibodies bind to distinct sites on the SARS-CoV-2 spike protein11 and were optimised by AstraZeneca with half-life extension and reduced Fc receptor and complement C1q binding.12 The half-life extension more than triples the durability of its action compared to conventional antibodies;13-15 data from the Phase III PROVENT trial show protection lasting at least six months.16 The reduced Fc receptor binding aims to minimise the risk of antibody-dependent enhancement of disease - a phenomenon in which virus-specific antibodies promote, rather than inhibit, infection and/or disease.17
Both Cilgavimab and Tixagevimab bind to different epitopes of the spike protein’s receptor-binding domain (RBD)1, which is important when designing a combination therapy so that mutations in the spike protein may not alter binding affinities for both antibodies- hopefully one one antibody would be affected while the other remains. In short, if a mutation leads to loss of binding for one antibody the other one will likely hold up and still neutralize the virus.

Unlike prior monoclonal antibodies, which are usually provided during an active infection as a therapeutic, Evusheld has several modifications made to the antibody in order to increase its half-life,2 which is the time it takes for half of a drug to be eliminated from the body. An increased half-life means that Evushield sticks around for longer, supposedly being able to target the virus as soon as SARS-COV2 infects an individual.
So unlike other monoclonal therapeutics Evusheld may last up to 9 within the body with a possibility of lasting up to 12 months.

Also, Evusheld was developed to be provided intramuscularly unlike other monoclonals, meaning that Evusheld can be provided quickly without the need to wait several hours as is needed for intravenous administration of other monoclonals. This may make Evusheld more appealing for those who may not want to wait hours at a treatment clinic, or may be concerned about exposure to SARS-COV2 as is likely the case for immunocompromised individuals.
Emergency Use Authorization and Prioritized Groups
In December 2021, the FDA granted an EUA for Evusheld for use as a pre-exposure prophylactic (PrEP) for those 12 years and older weighing at least 40 kg. The combination therapy is provided at a dosage of 150 mg Cilgavimab and 150 mg Tixagevimab (300 mg Evusheld in total) given intramuscularly as two separate consecutive injections. This is recommended to be repeated every 6 months as needed.
However, the emergence of Omicron led the FDA to recommend doubling the dosage of each antibody (total of 600 mg Evusheld), and eventually another update was provided at the end of June 2022 in regards to the use of Evusheld.
Because of Evusheld’s use as a prophylactic, the combination treatment has been prioritized for high-risk individuals such as those who are immunocompromised.
As a reference3, people who are included as priorities for Evusheld are shown below.

For those overseas, Evusheld was approved for use in the UK and EU in March 20224:
As being one of the only prophylactic options available, Evusheld may be something worth considering for those who are at high-risk and may not respond properly to vaccination. Please remember to consult a medical professional for medical advice and whether Evusheld may be right for you.
Effectiveness against Ba.4/Ba.5
For the sake of relevance, I will limit the scope of Evusheld’s review to the current Omicron wave of Ba.4/Ba.5 and look at the available evidence against the current variant. If you would like a review of the clinical trials and nonclinicals for Evusheld please let me know. I’ll link a few of those studies below as well:
The SARS-CoV-2 monoclonal antibody combination, AZD7442, is protective in non-human primates and has an extended half-life in humans (Loo, et. al.)
Intramuscular AZD7442 (Tixagevimab–Cilgavimab) for Prevention of Covid-19 (Levin, et. al.)
Tixagevimab + Cilgavimab: First Approval (Keam, S. J.)
This review provides an overview of many of the clinical trials using Evusheld.
Unfortunately, with Ba.4/Ba.5, in vitro studies have shown that Evusheld may have reduced neutralizing capabilities against the Ba.4/Ba.5. spike protein.
One study from Aggarwal, et. al.5 utilized a neutralization assay using HAT-24 cells and live virus isolates along with monoclonal antibodies such as Evusheld. In this study, researchers found a drop in potency of Evusheld against Ba.5:
The currently clinically utilised monoclonal antibodies (mAbs), Evusheld and Sotrovimab, were assessed for neutralisation capacity primarily against the Omicron variants BA.2 and BA.5, as they currently represent the dominant variants within the community. These therapies may need to be used in individuals that have not mounted a vaccine response from therapeutic induced or pre-existing immunodeficiencies. Whilst activity was retained using Evusheld for BA.2, we did observe a drop in potency when testing BA.5.
The researchers comment that Evusheld overall showed a 14.3-fold reduction in neutralization against Ba.5 when compared to neutralization against Clade A (Wuhan).
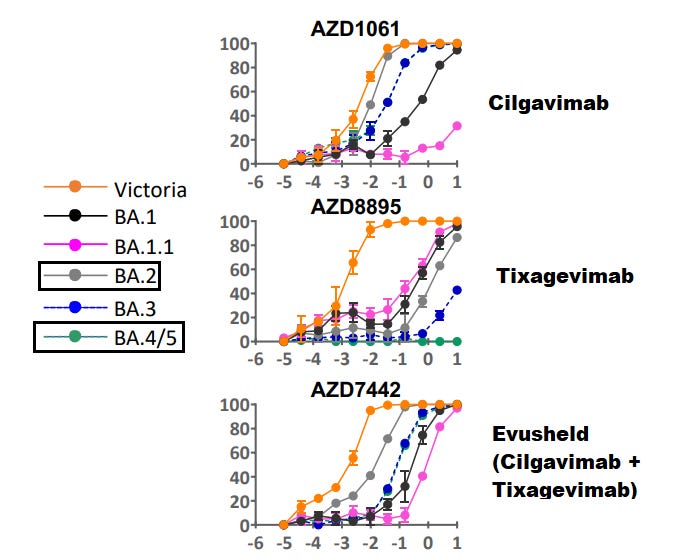
This can be seen with the following neutralization curves which provide interesting results:

Remember that Evusheld contains both Cilgavimab and Tixagevimab, which both target nonoverlapping epitopes. When we hear that Evusheld is not as effective, we should ask ourselves which antibody may be the cause, and why that could be the case with different variants.
When reading the neutralization curves above, note that the x-axis shows higher concentrations of the monoclonal antibody. Generally, the higher the concentration needed for neutralization the less effective the antibody has become against the variant spike. In reading the curves, this usually means that as the curves shift more to the right the less effective the antibody is at neutralizing that variant. We can see that with the Ba.5 curve being positioned more to the right compared to Clade A’s curve as an example.
As you can see, in this neutralization assay Evusheld (Fig. 1J) required a higher concentration to reach full neutralization against Ba.5 spike (purple) compared to Ba.2 (lime green) and Wuhan (orange). However, we can see that Tixagevimab (Fig. 1L) lost much of the activity against Ba.2 and Ba.5, suggesting that most of the neutralizing activity of Evusheld may be derived from Cilgavimab (Fig. 1K), which itself appears to have lost some of its neutralizing capacity against Ba.5. In essence, Cilgavimab is likely providing neutralization against much of the Omicron sub-lineages such as Ba.2 and Ba.5, with some of the neutralization being lost against Ba.5. Note that these results are associated with these neutralization assays, and the results may be different from other assays based on methodologies.
The researchers provide some explanation as to why this may be happening with Ba.5 (emphasis mine):
All Omicron variants contain significant substitutions within their RBD site relative to the ancestral variant. Therefore, fluctuations in potency in antibodies against the RBD (e.g. Evusheld) are to be expected. Furthermore, the Evusheld binding sites cover the unique RBD polymorphisms (L452R, F486V, and R493Q) that distinguish the BA.5 spike glycoprotein from the BA.2 spike.
Another preprint from Tuekprakhon, et. al.6 provides some conflicting results with respect to Tixagevimab. In this study researchers utilized pseudoviruses and constructed neutralization curves. Interestingly, this study did not show complete loss of neutralization of Tixagevimab (AZD8895) against Ba.2 (grey). However, against Ba.4/Ba.5 (green) Tixagevimab did lose all neutralization activity.
The researchers suggest an 8.1-fold reduction in neutralization as compared to Ba.2 [context added]:
For AstraZeneca AZD1061 [Cilgavimab], activity to BA.4/5 was similar to BA.2 (< 2-fold reduction), whilst for AZD8895 [Tixagevimab] residual activity against BA.2 was knocked out. The activity of the combination of both antibodies in AZD7442 [Evusheld] (Dong et al., 2021) was reduced 8.1-fold compared with BA.2.
So on one hand the neutralization curve from Aggarwal, et. al. showed that Tixagevimab lost neutralization against Ba.2, yet this neutralization curve from Tuekprakhon, et. al. suggest otherwise.
So why the difference? It could be due to the difference in use of live virus and pseudovirus in these assays which may have influenced the results, although without further comparative evidence it may be difficult to figure out the differences. Regardless, Ba.4/Ba.5 are relevant to the current wave and the evidence in both studies suggest that Tixagevimab has lost all neutralization activity against the current Omicron wave.
That leaves us with the the question of why this is happening. It appears that this may be a consequence of the F486V7 mutation, which I originally thought would not have a big detrimental effect compared to the other mutations. However, emerging evidence suggests that the F486 residue creates a necessary hydrophobic pocket which can interact with hydrophobic amino acid residues found on the paratopes of antibodies (Dong, et. al.):
The AZD8895 interaction with the RBD F486 residue is distinctive. The RBD aromatic residue interacts extensively via a hydrophobic effect and van der Waals interactions with a hydrophobic pocket formed between the AZD8895 heavy and light chains (residue P99 of the heavy chain and an ‘aromatic cage’ formed by five aromatic side chains) (Fig. 1d and Extended Data Fig. 2a,b). A hydrogen bond (H-bond) network, constructed with 4 direct antibody–RBD H-bonds and 16 water-mediated H-bonds, surround residue F486 and strengthen the antibody–RBD interaction (Extended Data Fig. 2c).
So it is likely that interactions between hydrophobic, aromatic amino acids located on the spike RBD (the F486 residue) also interact with hydrophobic, aromatic amino acids that serve as part of the paratope of many antibodies. The mutation from an aromatic hydrophobic Phenylalanine (F) residue to a much smaller Valine (V) likely disrupts the hydrophobic pocket and pi-stacking from the aromatic residues, greatly reducing the binding affinity (check Footnote 7 below for comparisons of amino acid structures).
This can be seen in a model by Tuekprakhon, et. al., which shows that hydrophobic, aromatic residues of Tryptophan (W98), Tyrosine (Y92) and Phenylalanine (F101) from Tixagevimab’s paratope interact with the F486 residue of the spike’s RBD. Once again, the mutation F486V is likely to have disrupted this favorable interaction, reducing Tixagevimab’s binding affinity and causing the loss of neutralization.

Safety and Adverse Events
As with all therapeutics the safety should be considered, as well as the efficacy. In most clinical trials Evusheld has been considered well-tolerated, although there have been a few noteworthy adverse events (Kotton, C. N):
Although rates of serious adverse events were generally similar between tixagevimab/cilgavimab and placebo, there has been some concern as a higher proportion of persons who received tixagevimab/cilgavimab (0.6%) versus placebo (0.2%) in the PROVENT (Phase III Double-blind, Placebo-controlled Study of AZD7442 for Pre-exposure Prophylaxis of COVID-19 in Adult) trial reported cardiac adverse effects, including myocardial infarctions, arrhythmias, and cardiac failures; all of these persons had cardiac risk factors and/or a history of cardiovascular disease at baseline (1). In the STORM CHASER (Phase III Double-blind, Placebo-controlled Study of AZD7442 for Post- Exposure Prophylaxis of COVID-19 in Adults) trial of postexposure prophylaxis with tixagevimab/cilgavimab, no cardiac events were noted, although the cohort was younger and had fewer cardiac risk factors. Overall, the benefit of additional protection for immunocompromised patients seems to outweigh the potential risk for cardiac events.
Additional adverse events can be found in the Keam, S. J. review, including possible anti-Evusheld antibodies being formed, although no evidence is provided to the actual toxicity of such anti-Evusheld antibodies:
Through day 183 after tixagevimab + cilgavimab administration in the PROVENT trial, treatment-emergent anti-tixagevimab, anti-cilgavimab and anti-tixagevimab + cilgavimab antibodies were detected in 0.8% (6/716), 1.1% (7/644) and 1.3% (10/743) evaluable participants. Anti-drug antibodies do not appear to have any impact on the efficacy or safety of tixagevimab + cilgavimab [12].
Like with all medications, if one were to consider Evusheld ask about possible side effects and adverse events.
In Consideration of Evusheld
With renewed concerns over Ba.4/Ba.5, it’s important to examine what may or may not work within the current wave. Here, we took a look at the new combination monoclonal therapy Evusheld that serves as the first form of PrEP against SARS-COV2. We also looked at whether it holds up within the current Ba.4/Ba.5 wave.
This review was not intended to be exhaustive, and if it is still considered too heavy consider the key points below:
Evusheld is a combination prophylactic for use in the prevention of SARS-COV2.
Evusheld combines up to 300 mg of Cilgavimab and 300 mg Tixagevimab provided intramuscularly with routine administration considered every 6 months. The EUA for Evusheld is allowed for those over the age of 12 weighing at least 40kg.
Unlike other monoclonal therapeutics, Evusheld is suspected to last up to 9 months and possibly up to a year due to an extended half-life.
Priority for Evusheld is given to those at high-risk, non-responsive to vaccines and those who are immunocompromised. If you are at high-risk of a severe COVID infection you may be suitable for Evusheld.
With Ba.4/Ba.5, Evusheld appears to have reduced neutralizing capabilities, although it is expected to hold up and provide protection against the current wave. Further evidence and studies should be conducted to provide clearer evidence.
The reduced neutralization of Evusheld is predominately due to Tixagevimab and the F486V mutation, leading to a complete loss of neutralization of Tixagevimab against Ba.4/Ba.5 spike. This suggests that Cilgavimab is the active antibody within the combination.
Evusheld may be provided with other monoclonal therapeutics. If one becomes infected while on Evusheld, he/she may be able to consider other monoclonal therapies in conjunction with Evusheld.
ALWAYS consult with medical professionals for proper advice and information. Use this information for knowledge-purposes, not as medical advice.
As of now messaging appears to be limited on the use of Evusheld, meaning that many vulnerable populations may not have access to treatment options- something that has been routine during the pandemic (Kotton, C. N):
Unlike with COVID-19 vaccines, there has not been large-scale support for developing methods for tixagevimab/cilgavimab distribution across many different communities and populations. We need to establish and ensure methods for more equitable distribution, with access for all regardless of health and social economic disparities. Variation in allocation across the different states has been dramatic. Furthermore, we need to provide guidance as to how to best establish a centralized and coordinated process for distribution and organize physical infrastructure for safe and efficient administration.
If there are concerns about the most vulnerable being hit hard by the next wave, it may be imperative that messaging creates awareness of Evusheld as a possible option for those who would likely benefit from such an option. This includes making more doctors and medical professionals aware of its existence, so that those who may need it the most may not go wanting.
If you enjoyed this post and other works please consider supporting me through a paid Substack subscription or through my Ko-fi. Any bit helps, and it encourages independent creators and journalists outside the mainstream.

For those who would like to know more about the actual interaction between the antibodies and the RBD, here’s an excerpt from Dong, et. al.:
AZD8895 binds to the receptor-binding ridge of RBD, and AZD1061 binds to one side of the RBD edge around residue K444 and the saddle region of the receptor binding motif RBM), both partially overlapping the ACE2 binding site (Fig. 1a,,bb,,c,c, ,2a2a–b). These features explain the competition between the antibodies and ACE2 for RBD binding from our previous study, e.g., both AZD8895 and AZD1061 neutralize the virus by blocking RBD access to the human receptor ACE217. Aromatic residues from the AZD8895 heavy and light chains form a hydrophobic pocket that surrounds RBD residue F486 and adjacent residues (G485, N487) (Fig. 1a, ,1d;1d; Extended Data Fig. 2a–c). This mode of antibody-antigen interaction is unusual in that the formation of the antibody pocket is caused by wide spatial separation of the HCDR3 and LCDR3. Overlays of the substructure of RBD in complex with AZD1061 (Fig. 2c) and the structure of RBD in complex with both AZD8895 and AZD1061 (Fig. 2d) suggest that AZD1061 is able to bind RBD in both “up” and “down” conformations of the S trimer. We compared the RBD/AZD1061 crystal structure with the published cryo EM structures of the human mAbs C119 and C13513. AZD1061, C119, and C135 have overlapping but different epitopes, as R346 and K444 of the RBD are key residues for AZD1061 and C13522 binding but were not important for C119 binding (Extended Data Fig. 5e).
Dong, J., Zost, S. J., Greaney, A. J., Starr, T. N., Dingens, A. S., Chen, E. C., Chen, R. E., Case, J. B., Sutton, R. E., Gilchuk, P., Rodriguez, J., Armstrong, E., Gainza, C., Nargi, R. S., Binshtein, E., Xie, X., Zhang, X., Shi, P. Y., Logue, J., Weston, S., … Crowe, J. E., Jr (2021). Genetic and structural basis for SARS-CoV-2 variant neutralization by a two-antibody cocktail. Nature microbiology, 6(10), 1233–1244. https://doi.org/10.1038/s41564-021-00972-2
A few of the amino acid changes for the antibodies can be seen below (Levin, et. al.):
These antibodies contain the half-life–extending M252Y/S254T/T256E (YTE) modification20 and the L234F/L235E/P331S (TM) modification that decreases binding of the Fc receptor and complement component C1q.21,22
Remember that the base of the antibody (the stalk, or Fc) binds to the Fc receptor on the surface of cells. This binding stimulates phagocytic and cytotoxic cells in order to remove microbes. Reducing the binding of the Fc receptor likely reduces the targeting and elimination of these monoclonals, meaning they can circulate within the body for much longer.
One in vitro study from Loo, et. al. shows the reduced binding affinity of each antibody towards the Fc receptor. Wildtype refers to the antibodies without modifications, showing that the wildtype antibodies have higher binding to Fc receptors in the graph below:
Levin, M. J., Ustianowski, A., De Wit, S., Launay, O., Avila, M., Templeton, A., Yuan, Y., Seegobin, S., Ellery, A., Levinson, D. J., Ambery, P., Arends, R. H., Beavon, R., Dey, K., Garbes, P., Kelly, E. J., Koh, G., Near, K. A., Padilla, K. W., Psachoulia, K., … PROVENT Study Group (2022). Intramuscular AZD7442 (Tixagevimab-Cilgavimab) for Prevention of Covid-19. The New England journal of medicine, 386(23), 2188–2200. https://doi.org/10.1056/NEJMoa2116620
Loo, Y. M., McTamney, P. M., Arends, R. H., Abram, M. E., Aksyuk, A. A., Diallo, S., Flores, D. J., Kelly, E. J., Ren, K., Roque, R., Rosenthal, K., Streicher, K., Tuffy, K. M., Bond, N. J., Cornwell, O., Bouquet, J., Cheng, L. I., Dunyak, J., Huang, Y., Rosenbaum, A. I., … Esser, M. T. (2022). The SARS-CoV-2 monoclonal antibody combination, AZD7442, is protective in nonhuman primates and has an extended half-life in humans. Science translational medicine, 14(635), eabl8124. https://doi.org/10.1126/scitranslmed.abl8124
Kotton C. N. (2022). Belt and Suspenders: Vaccines and Tixagevimab/Cilgavimab for Prevention of COVID-19 in Immunocompromised Patients. Annals of internal medicine, 175(6), 892–894. https://doi.org/10.7326/M22-1026
Keam S. J. (2022). Tixagevimab + Cilgavimab: First Approval. Drugs, 82(9), 1001–1010. https://doi.org/10.1007/s40265-022-01731-1
SARS-CoV-2 Omicron BA.5: Evolving tropism and evasion of potent humoral responses and resistance to clinical immunotherapeutics relative to viral variants of concern
Anupriya Aggarwal, Anouschka Akerman, Vanessa Milogiannakis, Mariana Ruiz Silva, Gregory Walker, Andrea Kidinger, Thomas Angelovich, Emily Waring, Supavadee Amatayakul-Chantler, Nathan Roth, Germano Coppola, Malinna Yeang, Tyra Jean, Charles Foster, Alexandra Carey Hoppe, C. Mee Ling Munier, David Darley, Melissa Churchill, Damian Starck, Daniel Christ, Gail Matthews, William Rawlinson, Anthony D. Kelleher, Stuart Turville
medRxiv 2022.07.07.22277128; doi: https://doi.org/10.1101/2022.07.07.22277128
Further antibody escape by Omicron BA.4 and BA.5 from vaccine and BA.1 serum
Aekkachai Tuekprakhon, Jiandong Huo, Rungtiwa Nutalai, Aiste Dijokaite-Guraliuc, Daming Zhou, Helen M. Ginn, Muneeswaran Selvaraj, Chang Liu, Alexander J. Mentzer, Piyada Supasa, Helen M.E. Duyvesteyn, Raksha Das, Donal Skelly, Thomas G. Ritter, Ali Amini, Sagida Bibi, Sandra Adele, Sile Ann Johnson, Bede Constantinides, Hermione Webster, Nigel Temperton, Paul Klenerman, Eleanor Barnes, Susanna J. Dunachie, Derrick Crook, Andrew J Pollard, Teresa Lambe, Philip Goulder, OPTIC consortium, ISARIC4C consortium, Elizabeth E. Fry, Juthathip Mongkolsapaya, Jingshan Ren, David I. Stuart, Gavin R Screaton
bioRxiv 2022.05.21.492554; doi: https://doi.org/10.1101/2022.05.21.492554








While I do see absolute value in the use of Evusheld as a prophylactic for immuno-compromised individuals, I still have several questions in general. I have been a bit stuck on secretory IgA for a while. I see people claiming that is the reason the vaccines are useless is because the vaccines promote the production of IgG or IgM antibodies and not IgA. Considering that the virus enters via the respiratory tract, then having SIgA would be the best candidate to neutralize the virus prior to it binding ACEII and gaining entry into our cells. Again, I have seen studies which claim that Pfizer's vaccine did indeed illicit the creation of SIgA (as did natural infection). Yet, I wouldn't claim that the vaccine induced antibodies are doing a great job at protecting from infection (maybe this is just immune escape caused by variants or maybe the vaccine created spike is actually too different from natural spike due to the optimization done by pharmaceutical companies). Also, with the neutralization assays used they are literally combining things in a test tube and saying "look, it bound" - it doesn't necessarily mean that these antibodies/antigens will come into contact with each other in the body).
As Evusheld is an intramuscular shot of mABs which would quickly enter the bloodstream and circulate for a pretty long time (and be well tolerated by the body as they do appear to be a normal thing seen in the human body). Note, I just said an IM shot will quickly enter the bloodstream - yes, I just looked that up (https://www.ncbi.nlm.nih.gov/books/NBK556121/) - so total lie, that the vaccine just stays in those cells around the injection site. But I digress, I am wondering if (and I presume Evusheld is IgG isotype) the IgG antibodies wouldn't be considered neutralizing, but rather more as a defense against infected cells as IgG can activate the complement system destroying infected cells.
Sorry for this stream of consciousness comment - there are just so many questions and so few actually useful answers and I just have this desire for all of this to make sense (and it just doesn't). One last comment - I dug up the federal contract award for this drug... just to see how much it cost: https://govtribe.com/award/federal-contract-award/other-transaction-agreement-w911qy2190001
Personally, I'd rather take my herbal combo of immune support, Astragalus, Eleuthero, Rhodiola and Antivirals Chinese skullcap, kudzu, Isatis. There's others but these are my core group