Taking the time to smell the roses...and rose-smelling products.
In lieu of Mother's Day let's examine where roses get their distinct, floral scent from.
I have smelled the roses…and I don’t like them
Mother’s Day is just around the corner, and just like every year I get many of the mothers in my life the perfect gift- candles!
Maybe not the perfect gift, but so far I haven’t had any complaints and most mothers seem to like candles. 🤷♂️
So a few days ago I scuttled off to grab some candles, wandering around a Yankee Candle store huffing and snuffing any candles that I could. I’m partially inclined to think that the employees thought I was some vagabond, and I wouldn’t blame them.
But anyhow, one of the employees caught me mid-sniff with my nose deep into a candle. She told me that I shouldn’t actually smell the candle, but the lid instead. The volatile compounds from the candle will get trapped in the lid and supposedly provides a better smell profile for when you burn the candle.
I thought this wouldn’t really make a difference, but when trying it out myself there were very distinct smells between the lids and the actual candle; so much so that I put back one or two because of the stronger smell in the lid. This may also suggest that candles shouldn’t be left with their lids off or else all of their volatile compounds will escape and leave you with a stale candle.
Candles owe their distinct fragrances to the organic compounds that reside within their wax. Many have various degrees of volatility, but when burned the heat helps to release these compounds into the air and provides the symphony of compounds that, hopefully, help fill your house with an appealing scent.
But this isn’t exactly about candles. Suffice it to say, I generally do not like the scent of floral candles. I find them to be far too strong, but also strangely chemical in nature. I’m generally sensitive to smells- whether I leave with candles or not I’m guaranteed to leave a YK store with a headache. If I grabbed any floral candles they generally have to be muted in some degree.
Now, this wouldn’t be a story about roses without smelling actual roses. Recently, I took a walk with a good friend through a flower garden. When available, we would stop and smell whatever flowers were still in bloom, with some either having no smell or mild, floral notes (my kind of flowers!).
But as we explored further we came across a rose garden barely coming to life in flowers. Even as barren in blossoms as the garden was, there were still a few in bloom.
And so, as the saying goes, we stopped to smell the roses.
I guess I don’t do this often, and unfortunately upon sniffing the roses I didn’t come out of it with a pleasant experience. Rather, I found the smell to be strangely too chemical, far too close of a resemblance to the candles I was huffing a few days prior.
Of course, roses should smell like roses. It’s not as if the botanists and garden workers were spraying down roses with artificial rose-scents…at least I don’t think so. I would argue that my aversion to the smell of natural roses may be, in part, due to how overused and ubiquitous the smell is with nearly all forms of cosmetics and air fresheners smelling predominately of roses.
After my disappointing sniff, I commented to my friend that the smell had a slight hint of woody chemicals (more of a chemical smell that smells…chemical to be honest), and remarked that maybe the smell came from some sort of terpene, as many of the woodsy smells from pine trees are derived from terpenes.
This actually got me thinking of what compounds are responsible for the smells that I have unfortunately not grown fond of.
A Rose Oxide by any other chirality
Nearly all of the rose-smelling things we come across are based on a select few compounds. In this case, the compound in question is called rose oxide, which is a terpene oxide with the following structure:

Rose oxide is a rather interesting compound, because it actually includes 4 variations of the above molecule. The only difference lies within the orientation of the molecule’s atoms, but that seemingly insignificant detail makes all the difference in how we sense these compounds.
In organic chemistry there’s a concept known as chirality, or “handedness”. Most people describe molecules without recognizing that the position of the atoms in a molecule are actually pivotal to a molecule’s nature.
As an example of chirality, take a look at both your left and right hands. By all accounts, they should look very similar- 4 fingers, one thumb, palms that face inward as well as fingers that curl inward as well. In this regard, the makeup of your left and right and should be nearly the exact same.
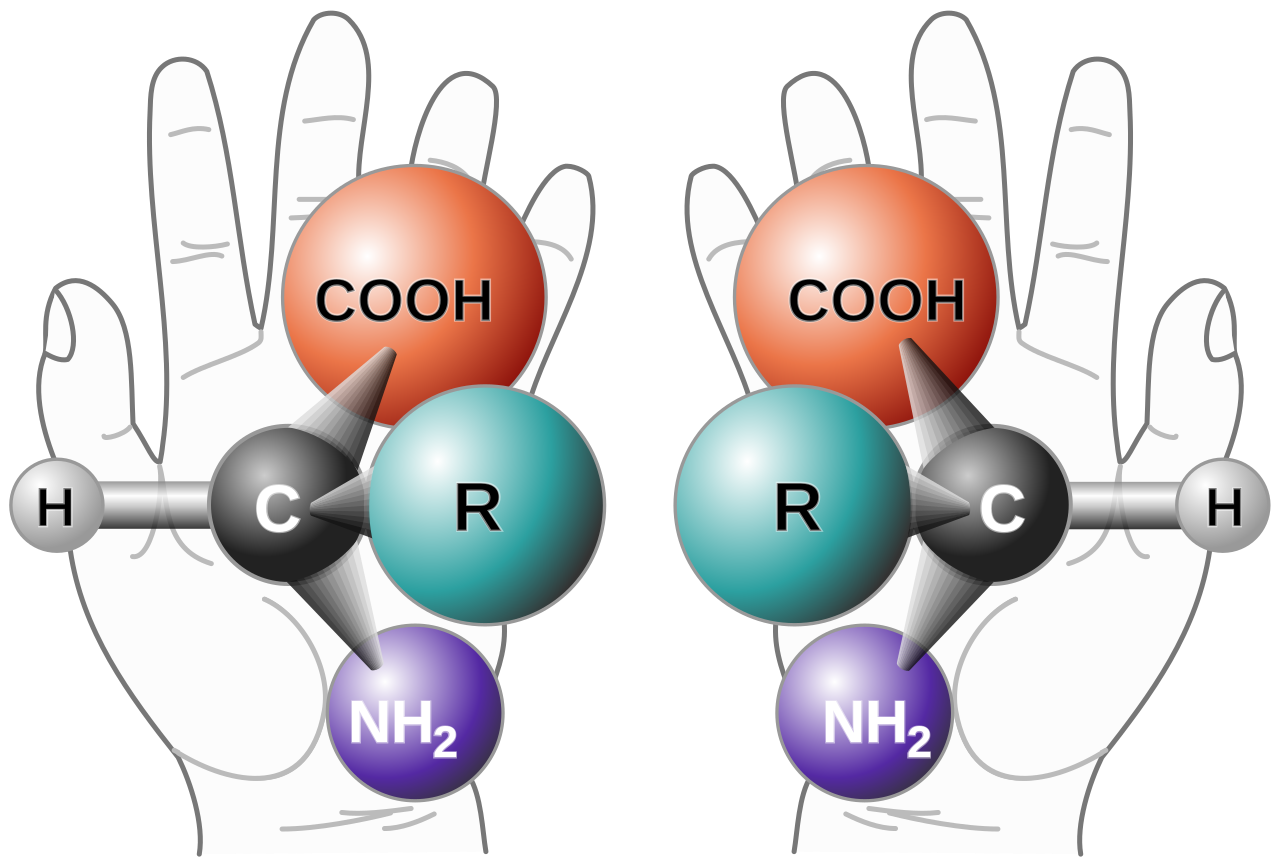
However, what happens when you try putting a left-hand glove on your right hand? The fit doesn’t work well, possibly because the bends in the glove go opposite your joints. But regardless, you can tell when you put a glove on the wrong hand, or if you put a shoe on the wrong foot.
That’s because our hands are essentially “chiral”, as in our hands are not superimposable onto each other. Try overlapping your hand sand your thumbs will stick out on opposite sides while the wrong fingers will lay on top of one another.
In this case, our hands and feet are actually enantiomers, or mirror opposites of one another.
Although they look nearly identical they don’t operate in the exact same fashion. Try twisting a doorknob with both your left and your right hand, and you’ll likely notice a difference in how your hand contorts.
Organic molecules work in the same fashion. Their orientations are critical for everyday life, such as whether a molecule binds to a receptor, or if a substrate can readily bind within the active site of an enzyme. The chemical makeup is only part of the process. How the atoms are positioned dictates whether a molecule may be in close proximity to the functional groups of other molecules including proteins, nucleic acids, or even lipids.
In the case of rose oxide, all 4 variations carry the exact same molecular structure shown above. The only difference is that at chiral regions the carbon atoms point in different directions. Again, these minor differences in orientation make a huge difference in smells.
Britannica provides a good, brief video explaining rose oxide. Unfortunately, the video cannot be embedded so I refer you to the link below:
Learn about the different sources that give a rose its distinct smell- Britannica
All four orientations of rose oxide are referred to as stereoisomers due to having similar structures but different orientations, with the term enantiomers being reserved for molecules that are mirror-images of one another.
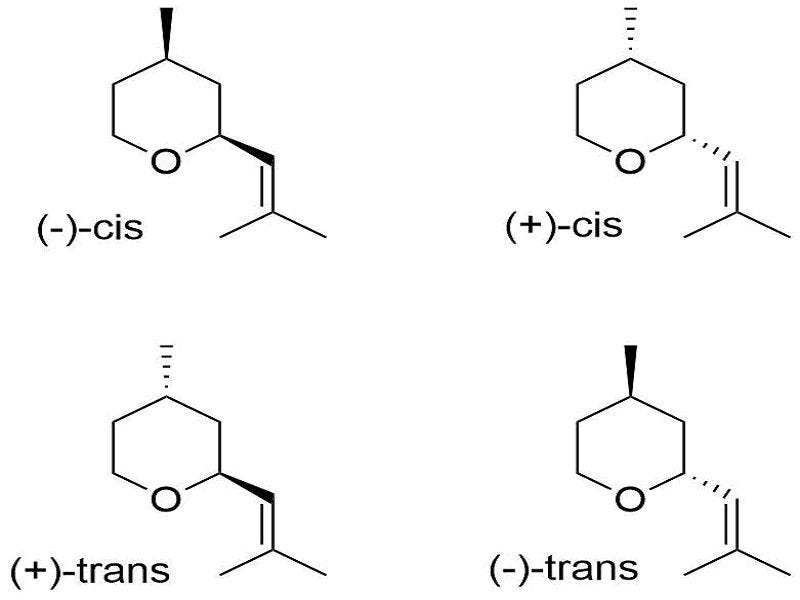
Here, the differences in shading within each stereoisomer denote the direction that group is pointing. For instance, the dark, bold shading tells us that group is pointing towards us/forward. The dashed lines tell us that those groups point backwards/away from us.
If you’re curious how we have 4 stereoisomers, there are two chiral carbons in this rose oxide (can you figure out where?). That means that functional groups attached to these chiral carbons can either point forwards or backwards, giving us two different orientations around one chiral center. Two chiral centers with two possible orientations gives us a total of 4 possible stereoisomers.
Interestingly, not all 4 of these isomers provide the same profile. As noted in the Britannica video the biggest offender (in my opinion) is (2S, 4R)- rose oxide, which is the (—)-cis-rose oxide shown above in the top-left corner.
This isomer in particular appears to be the biggest contributor to the pungent smell of roses, although all 4 contribute in some fashion, and it's likely the combination of these stereoisomers in partnership with binding to olfactory receptors that produces the unique smell that makes its way into our products.
As you can imagine, the “floral” notes in fruits are also related to this isomer. Various grapes and wines contain this (-)-cis rose oxide1, as do more floral fruits such as lychees.2
This is likely because rose oxide is derived from the compound citronellol, a monoterpenoid found in many plants and used in many products for its sweet smell. It acts as the main compound in citronella oil.
Note the structural similarities, in which citronellol is a terpene alcohol in contrast to rose oxide being a terpene oxide:

Take some time to smell the (2S, 4R)- rose oxide
So maybe I’m not appreciative of the smells of roses due to how often its used. Manufacturers generally take citronellol and synthesize rose oxide from that compound for use in cosmetics, candles, and air fresheners.
But even if I cannot appreciate the smell of roses, the smell is popular for a reason, and in many cases are, hopefully, synonymous with joyful memories and pleasantries, such as the ones associated with Mother’s Day.
In any given case, a Happy Mother’s Day to all the mothers out there! I hope your day is filled with all of the (2S, 4R)- rose oxides that you can smell!

Substack is my main source of income and all support helps to support me in my daily life. If you enjoyed this post and other works please consider supporting me through a paid Substack subscription or through my Ko-fi. Any bit helps, and it encourages independent creators and journalists such as myself to provide work outside of the mainstream narrative.

Luan, F., Mosandl, A., Gubesch, M., & Wüst, M. (2006). Enantioselective analysis of monoterpenes in different grape varieties during berry ripening using stir bar sorptive extraction- and solid phase extraction-enantioselective-multidimensional gas chromatography-mass spectrometry. Journal of chromatography. A, 1112(1-2), 369–374. https://doi.org/10.1016/j.chroma.2005.12.056
Ong, P. K., & Acree, T. E. (1999). Similarities in the aroma chemistry of Gewürztraminer variety wines and lychee (Litchi chinesis sonn.) fruit. Journal of agricultural and food chemistry, 47(2), 665–670. https://doi.org/10.1021/jf980452j




Just sayin, the candles at YK are probably synthetic chemical scented rather than essential oil of rose (way pricey) scented, setting up your limbic system to recoil at the actual rose aroma. Also, rose gardens are generally sprayed with toxic chemicals for mites, aphids, and other critters that like to eat roses, which will leave you less than pleasantly informed. The roses in florist shops from South America are so toxic, a friend who purchased a florist ship broke put on a rash and had to find organic suppliers
So when you smell the roses, find some organic and or unsprayed specimens. You might be amazed.
very interesting.
could identify the natural (strong preference) rose scent to synthetic blindfolded 100%, nose just knows.
always wondered about the variety of natural rose & flower scents, ranging from jasmine like, citrusy, clove like, fruity, minty…there are so many… have a salvia which has flowers that smell like Concord grapes, a rose that smells like apricots (Lady Hillingdon), once had another rose that smells like candy canes (Portadown Fragrance)…