Remarks on the South Korean study on vaccine-related myocarditis
And how issues with global surveillance and diagnoses of VRM may misreport actual adverse events.
A few days ago a new study was released from South Korea1 noting the prevalence of myocarditis-post vaccination among the country’s population.
This study was circulated by way of Rav Arora’s Substack article covering this study:
Since Arora’s article goes over findings in the study, and since the study isn’t one in that requires extra scrutinizing (since it examines the data provided with respect to adverse events and compares them based on demographic data and number/type of vaccinations), I suggest people look at his post for information not covered here.
This study, in short, further emphasizes continuous findings noting increased risk of myocarditis post-COVID vaccination, especially for young men who receive the mRNA vaccines.
But what’s unique to South Korea, and unlike other countries, is the fact that South Korea established a more robust investigation into vaccine adverse events by way of their INFECTIOUS DISEASE CONTROL AND PREVENTION ACT.
The act makes it necessary for medical professionals to report any suspected or diagnosed cases of adverse events associated with vaccines:
And it also requires that the Korea Centers for Disease Control and Prevention (KCDCP) investigate any reports to figure out the cause of the adverse events, and determine whether they are caused by vaccines:
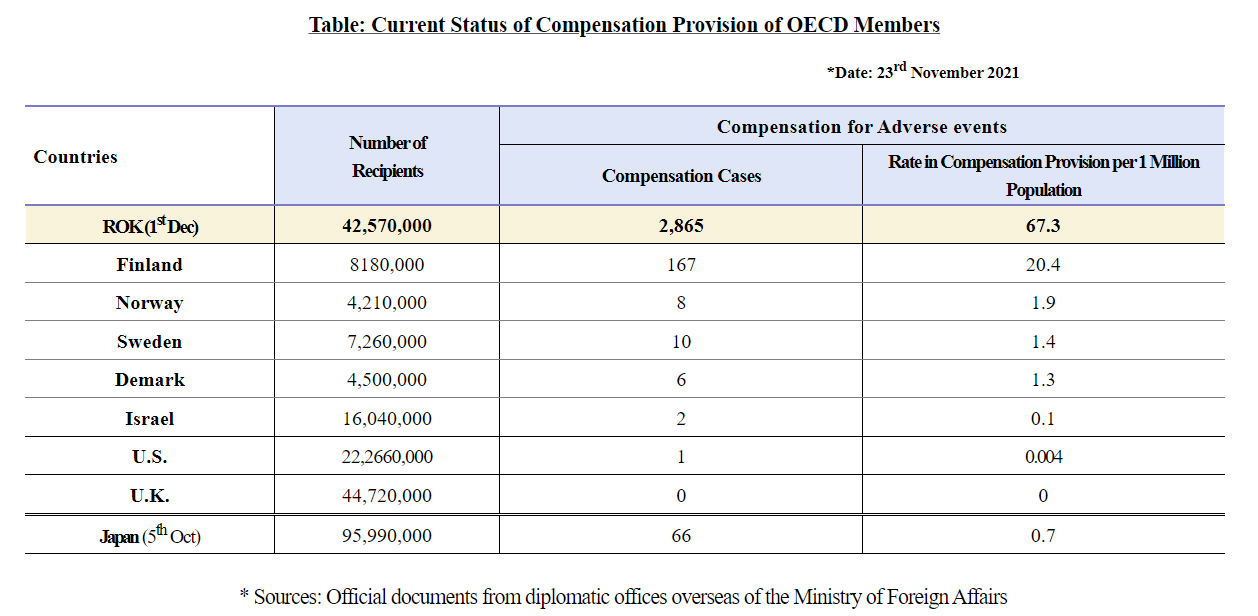
It should also be noted that South Korea leads the world in COVID-vaccine compensations:
At the time of the press release publication, South Korea was providing compensation at a rate of 67.3 compensations per million citizens (a little under 2,900 compensations made at that time).
In comparison, how is the US doing with respect to compensation? Supposedly, only one compensation was made towards a COVID vaccine victim as reported at the time of the press release.2
All this being said, South Korea’s monitoring of COVID vaccine adverse events provides a different perspective, and one that may shed more light on these adverse reactions aside from looking at US data predominately. They also seem to be the most proactive in elucidating actual risks and harms related to the vaccines.
What’s different with this study?
1. Inclusionary Time Periods
Now, aside from the bureaucratic aspect, when it comes to metrics of reporting and diagnostic criteria South Korea’s approach is different relative to other countries.
Note that acute myocarditis was considered in cases where myocarditis occurred within 42 days of vaccination:
Acute myocarditis developed within 42 days after COVID-19 vaccination was considered as COVID-19 VRM.
Remember that myocarditis has generally been considered to occur within days of vaccination, and so the use of a far broader time period is important to consider. The CDC’s early reports3 about myocarditis noted myocarditis reported within 7 days of a COVID vaccine, and is a much shorter time window.
And when diving into the literature, it becomes apparent that inconsistent cutoffs are used.
For instance:
The study from Yonker, et al.4 which noted unbound, full spike within vaccinated, adolescent males included a small cohort with a vaccination time range 1-19 days (median 4). In this case, the individuals included were based on patients hospitalized for myocarditis who were approached to be included in the study, so there is no concrete cutoff point per se and were based more on the events of myocarditis occurring.
The study from Bramada, et al.5 (which I have yet to fully analyze) used a cutoff point of 7 days for their cohort of myocarditis patients.
One study looking at Nordic countries (Karlstad, et al.6) published in 2022 used a cutoff point of 28 for a 28-day risk assessment, although they appear to also conducted a shorter 7-day assessment as well.
Because of these differences it’s important to consider the following:
There are likely to be cases of myocarditis related to vaccination not being picked up due to short time windows, as may be the case for many myocarditis-related studies available (underreporting).
A broader time period may increase the risk of including background noise and may overreport myocarditis cases (overreporting). The further out from an intervention the more likely background incidences will be picked up.
The risk of the second point is possible here, as the further out from the initial vaccination point an adverse reaction is the more difficult it is to link the two together.
However, in the case of South Korea, this concern may be assuaged due to the fact that reports seem to reiterate the point that myocarditis post-vaccination are likely to occur within days of vaccination. In this case, even though South Korea is using a much broader inclusionary time period most of the data also falls within typical cutoff ranges:
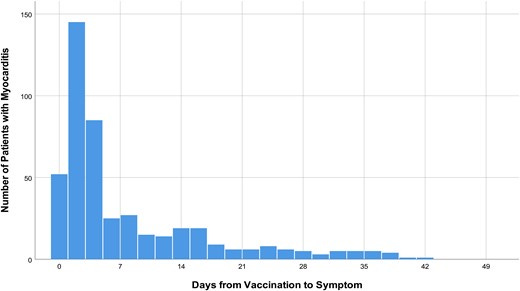
In any case, it’s important to consider the fact that time of inclusion is likely to influence overreporting or underreporting of myocarditis. It raises some necessary questions when cutoffs are extremely short and may miss out on actual cases of myocarditis. And in this case, the use of the broader time period doesn’t seem to to influence overreporting by Cho, et al, as their data seems to fall below other reports of VRM.
2. Diagnostic Criteria for Myocarditis
Another contrast to prior studies on myocarditis is the criteria used by Cho, et al, which seems to have been adopted by South Korea’s committee involved with evaluating each case of myocarditis.
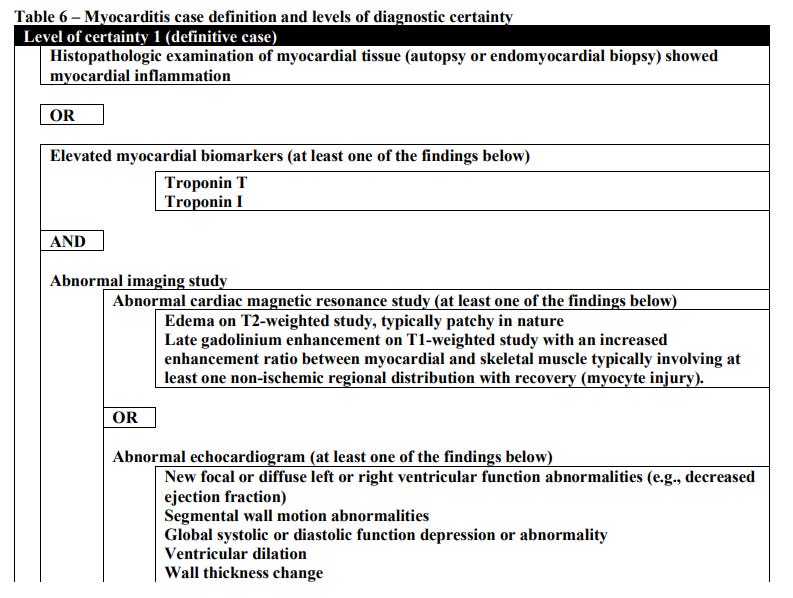
Here, the criteria seems to be based off of the Brighton Collaboration (BC). BC’s criteria falls along 3 categories in descending certainty:
Level 1 indicating a definitive case of myocarditis,
Level 2 being probable case,
and Level 3 being a plausible case.
According to the BC a Level 1 case of myocarditis is considered when histopathological findings or elevated biomarkers as well as imaging note myocardial injury akin to myocarditis. Symptoms aren’t included due to the assumption that testing for biomarkers and imaging would infer some sort of symptom onset.
Note that BC Level 3 was excluded in myocarditis evaluations, and BC Level 2 was included only if troponin levels were elevated and the patient did not test positive for COVID.
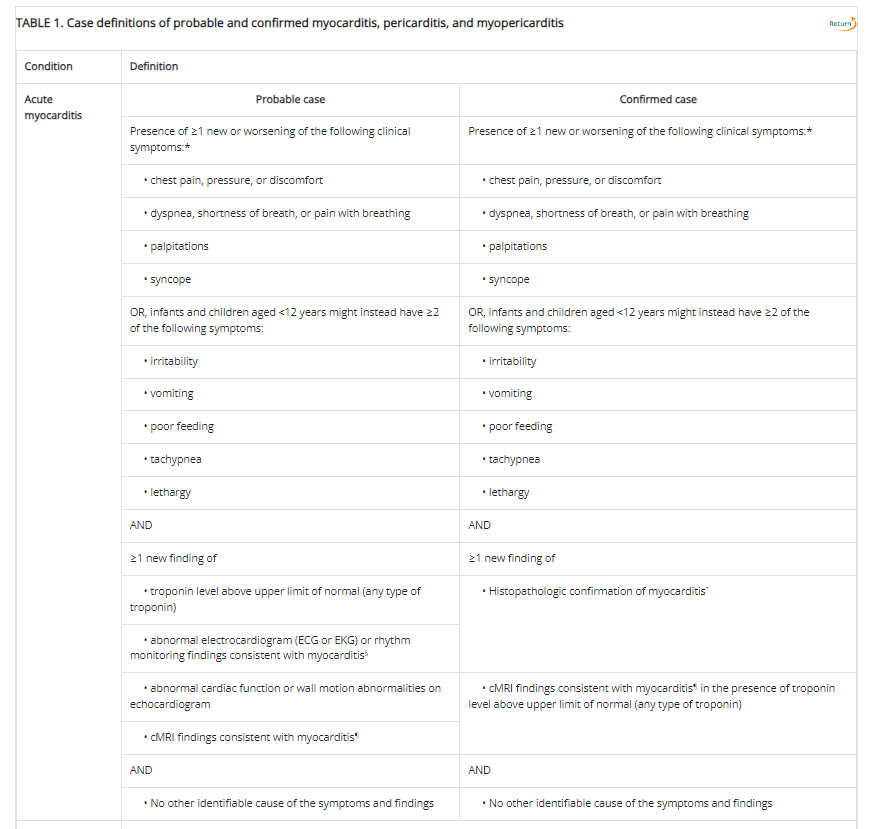
As a comparison, the CDC notes the following as a diagnostic criteria for myocarditis:
Aside from the exclusion of symptoms in the BC criteria due to assumed redundancy, the criteria used here are rather similar. This may suggest a similar criteria used by the CDC and Cho, et al. aside from the modified Level 2 criteria which appears to require both imaging and elevated troponin levels (the CDC’s definition requires one or the other for probable cases).
However, there is the fact that clinicians may use their own criteria when evaluating for myocarditis. This is more of a broader issue of how myocarditis is reported, given that there may not be consensus on how to report these cases, although a consensus diagnostic criteria may also miss out on possible cases that don’t present with consensus characterisits.
This is more of an aside, but it’s something worth considering when looking at different studies and discerning how they diagnoses myocarditis. Note that Cho, et al. raises points about the use of BC possibly leading to some forms of overreporting and some forms of underreporting when using this diagnostic criteria, and may lead to differences in reporting relative to how the CDC and other institutions define acute myocarditis.
Key Findings
As stated above, this study from Cho, et al. just further emphasizes what was already known in relation to the vaccines.
Most South Koreans received the mRNA-based vaccines (~71%) with another 25% receiving the AstraZeneca adenoviral vaccines.
COVID-19 VRM incidence was highest in mRNA-1273 vaccine [2.30 per 100 000 persons (95% CI, 1.95 to 2.69)] and followed by BNT162b2 [1.23 per 100 000 persons (95% CI, 1.10 to 1.38)], Ad26 (0.20 [95% CI, 0.04 to 0.58] per 100 000 persons), and ChAdOx1 [0.14 (95% CI, 0.08 to 0.22) per 100 000 persons] (Figure 2B). VRM incidence was significantly higher in mRNA vaccines than in other vaccines (1.46 [95% CI, 1.33 to 1.60] vs. 0.14 [95% CI, 0.08 to 0.23] per 100 000 persons, P < 0.001).
Reported cases of vaccine-related myocarditis (VRM) leaned predominately male (62.3%) and under the age of 40 (67.9%), following similar trends found in prior studies. In particular, rates of VRM were highest among men aged 12-17, and declined with each generational increase. This sex-discrepancy is also apparent among the second vaccination and biased more towards males, suggesting that possible differences in responses to the second vaccination may drive adverse reactions.
The incidence of VRM was highest in males between the ages of 12 and 17 years [5.29 (95% CI, 4.06 to 6.78) per 100 000 persons] followed by males between the ages of 18 and 29 years 2.93 (95% CI, 2.42 to 3.52) and lowest in females aged more than 70 years [0.16 (95% CI, 0.05 to 0.38) per 100 000 persons].
mRNA vaccines accounted for 96.3% of VRM cases, with most symptoms appearing within a few days of vaccination (median around 3 days with an IQR of 1-10 days.
Interestingly, VRM cases after the booster were far lower than with the second dose. However, this may be due to the fact any reported cases of VRM will filter people out from receiving additional doses. That is, the high rate of VRM after the second dose may remove those more predisposed to VRM in general, and thus would lead to a lower incident rate after the booster.
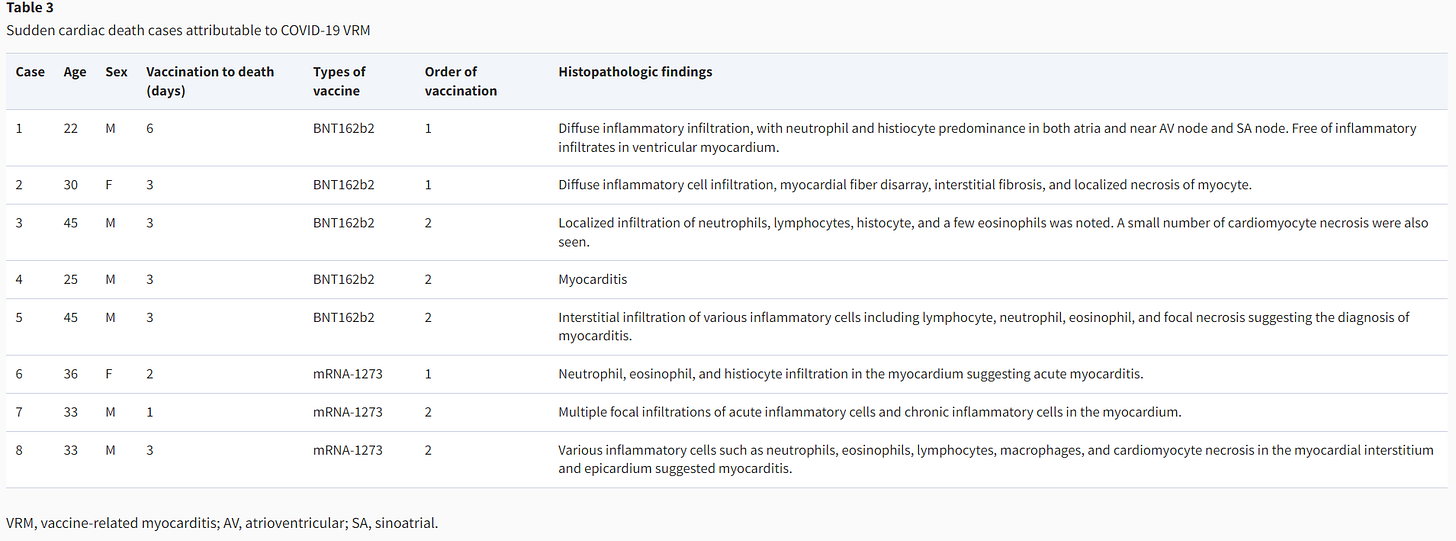
Probably one of the most important findings from Cho, et al. are the cases of sudden cardiac deaths. Cho, et al. reported on 8 cases of autopsies confirming VRM, with all deaths occurring within a week of vaccination, with the mRNA vaccines, and occurring predominately in younger males (2 were in females and 2 were in males in their 40s):
Note that neutrophils and lymphocytes make up a good portion of the histopathological findings, mimicking prior findings noting the presence of T-cells in the hearts of some deceased individuals, although in this case the characterization of the lymphocytes are not provided. A few tissues also noted eosinophilic infiltration and only one noting the presence of macrophages. There are also several cases of sudden cardiac death occurring after the 1st dose among the deceased as well.
Remember that the autopsy reports of two teenage boys reported by Gill, et al.7, as well as the case report autopsy of the man with Parkinson’s disease reported by Mörz, M8, noted eosinophilic infiltration in their autopsy findings. This is in contrast to Barmada, et al, which argues that eosinophilia does not appear to correlate with myocarditis (again, adding further rebuttal to their hypothesis given these findings in Cho, et al.).
These findings suggest that presentation of myocarditis are likely to differ between individuals, suggesting that more warnings should be looked out for when trying to catch possible cases of myocarditis as not all cases will present with similar pathological findings. More important, why these differences appear is something that requires further investigation, and still has yet to be elucidated.
What’s worth noting is that, in contrast to noted deaths related to COVID vaccination in South Korea’s database, the US’s VAERS system appears to lack any evidence of deaths related to the vaccines and were noted by Cho, et al. in their Discussion, with the explanation provided appearing to be due to incentives in reporting and the systems used between both countries (emphasis mine):
However, there was no death-related COVID-19 VRM in the largest cohort including 192 405 448 persons in the USA.3 In that report, they analyzed clinical events in 826 cases of myocarditis among younger than 30 years of age with detailed clinical information. Although we did not know the exact reason for this discrepancy between the two countries, the difference in the used case reporting system may be a possible explanation. Most studies from the USA used the VAERS,11 but this system is a passive reporting system that allows for underreporting or overreporting. On the contrary, the Korean government made a reporting system for all adverse events before starting COVID-19 vaccination and established a national compensation system for all medical expenses related to adverse events of COVID-19 vaccination. Besides these systems, because VRM was the special adverse reaction of COVID-19 vaccination with a legal obligation that should be reported to the KDCA, the risk of underreporting for VRM might be minimized.
Thus, the reliance on VAERS creates a serious detriment in investigative research. Because South Korea requires a more proactive approach in reporting, with an obligation to investigate myocarditis in particular, and was created prior to vaccine rollout in order to capture possible adverse reactions, there was more of an incentive to investigate adverse reactions in South Korea. In contrast, the use of VAERS and similar reporting systems may not provide the same proactive, obligatory approach here in the US, and thus deaths related to the vaccines may be overlooked if not reported accurate and if they do not spur further investigation.
Remember, research is only as good as the data that is used. It’s been rather apparent that US data relating to the COVID vaccines have been severely lacking, and with South Korea’s data it’s apparent that a more proactive approach should have been taken in order to capture and prevent adverse reactions, especially if it could reduce the risk of death associated with vaccination- something that still appears lacking in the US.
Overall, Cho, et al.’s data parallels those found in other studies. However, it’s also important to note that the overall rate of myocarditis here appears to be lower than other reports, and may suggest that the difference in diagnostic criteria may play a role. It’s also worth noting that genetic differences are likely to be at play as well, with South Korea being a more ethnically homogenous country.
In that regard, it’s worth noting that the discrepancy between sexes and myocarditis is not as strong as found in other countries such as the US, and rates of myocarditis don’t appear as low among those males within the age bracket of 40-65 as compared to other countries:
However, epidemiologic characteristics of COVID-19 VRM incidence in Korea were different from those of the previous studies: (i) male predominance seems to be weak in Koreans, (ii) no remarkable difference between the first and second vaccination dose, and (iii) COVID-19 VRM was not uncommon in individuals aged between 40 and 60 years.
Thus, it may be worth considering genetic, dietary, and cultural factors as playing a role in myocarditis. It’s curious why the predominance in males isn’t as prevalent in South Korea, and why adverse reactions may appear in in older males at a higher rate as well.
All this suggests that there’s still a lot left to be determined, and again points to the need for more transparency and better data in order to figure out what exactly is going on.
Substack is my main source of income and all support helps to support me in my daily life. If you enjoyed this post and other works please consider supporting me through a paid Substack subscription or through my Ko-fi. Any bit helps, and it encourages independent creators and journalists such as myself to provide work outside of the mainstream narrative.

Jae Yeong Cho and others, COVID-19 vaccination-related myocarditis: a Korean nationwide study, European Heart Journal, 2023;, ehad339, https://doi.org/10.1093/eurheartj/ehad339
Gargano JW, Wallace M, Hadler SC, et al. Use of mRNA COVID-19 Vaccine After Reports of Myocarditis Among Vaccine Recipients: Update from the Advisory Committee on Immunization Practices — United States, June 2021. MMWR Morb Mortal Wkly Rep 2021;70:977–982. DOI: http://dx.doi.org/10.15585/mmwr.mm7027e2external icon
Yonker, L. M., Swank, Z., Bartsch, Y. C., Burns, M. D., Kane, A., Boribong, B. P., Davis, J. P., Loiselle, M., Novak, T., Senussi, Y., Cheng, C. A., Burgess, E., Edlow, A. G., Chou, J., Dionne, A., Balaguru, D., Lahoud-Rahme, M., Arditi, M., Julg, B., Randolph, A. G., … Walt, D. R. (2023). Circulating Spike Protein Detected in Post-COVID-19 mRNA Vaccine Myocarditis. Circulation, 147(11), 867–876. https://doi.org/10.1161/CIRCULATIONAHA.122.061025
Barmada, A., Klein, J., Ramaswamy, A., Brodsky, N. N., Jaycox, J. R., Sheikha, H., Jones, K. M., Habet, V., Campbell, M., Sumida, T. S., Kontorovich, A., Bogunovic, D., Oliveira, C. R., Steele, J., Hall, E. K., Pena-Hernandez, M., Monteiro, V., Lucas, C., Ring, A. M., Omer, S. B., … Lucas, C. L. (2023). Cytokinopathy with aberrant cytotoxic lymphocytes and profibrotic myeloid response in SARS-CoV-2 mRNA vaccine-associated myocarditis. Science immunology, 8(83), eadh3455. https://doi.org/10.1126/sciimmunol.adh3455
Karlstad Ø, Hovi P, Husby A, et al. SARS-CoV-2 Vaccination and Myocarditis in a Nordic Cohort Study of 23 Million Residents. JAMA Cardiol. 2022;7(6):600–612. doi:10.1001/jamacardio.2022.0583
James R. Gill, Randy Tashjian, Emily Duncanson; Autopsy Histopathologic Cardiac Findings in 2 Adolescents Following the Second COVID-19 Vaccine Dose. Arch Pathol Lab Med 1 August 2022; 146 (8): 925–929. doi: https://doi.org/10.5858/arpa.2021-0435-SA
Mörz, M. (2022). A Case Report: Multifocal Necrotizing Encephalitis and Myocarditis after BNT162b2 mRNA Vaccination against COVID-19. Vaccines, 10(10), 1651. MDPI AG. Retrieved from http://dx.doi.org/10.3390/vaccines10101651