More on BioNTech's Lipid Nanoparticle Data
Examining Radiolabeled Study 185350 and the Possible Implications
When I examined one of BioNTech’s Studies (R-20-0072) I made a remark that using radiolabeled lipids would give a better examination of LNP distribution.
Fortunately for us BioNTech had the same idea and conducted another study (Study 185350) in which they deployed radiolabeled LNPs and examined which organs and tissues the LNPs were found in.
It’s a pretty straightforward study so we may not need to go into full detail, although some comparative analyses will be done. We also will speculate on what these results may suggest as well.
Examining Study 185350
The real name of this study is quite the mouthful, but it directly tells us what the purpose of this study is:
This study is deploying an LNP formulation that includes radiolabeled hydrogen.
Brief Overview of 3H-CHE and Radioactive Compounds
For a basic chemistry review, the atomic number of an element typically represents the number of protons found within the nucleus of an atom. Usually the number of neutrons in an atom balances the number of protons. However, the number of neutrons can sometimes vary. These types of atoms are referred to as isotopes. This usually occurs as you move down the periodic table and the nucleus of an atom grows larger. One hypothesis is that higher numbers of isotopes in larger atomic nuclei are a way of spacing out the high density of positively charged protons with additional neutrons.
Hydrogen is unique in that it typically has only one proton and no neutrons within its nucleus. However, some hydrogen isotopes may contain a few neutrons. In this case, the isotope used by BioNTech is referred to as tritium, which is a hydrogen atom with one proton and two neutrons within its nucleus.
Certain isotopes are unstable and release various forms of radiation in order to reach a typical atomic number.
The release of radiation is what provides researchers something to look for. More specifically, when a compound or drug is labelled with this radioactive isotope (hence the name “radiolabeled”) researchers are able to search for signs of radiation and see where their compound or drug has distributed, usually within an animal model such as mice or rats. The technique used in this study is called Liquid Scintillation Counting which measures photon emissions.
The radiolabeled compound deployed here is called Cholesteryl Hexadecyl Ether, [Cholesteryl-1,2-3H(N)] (3H-CHE for short).
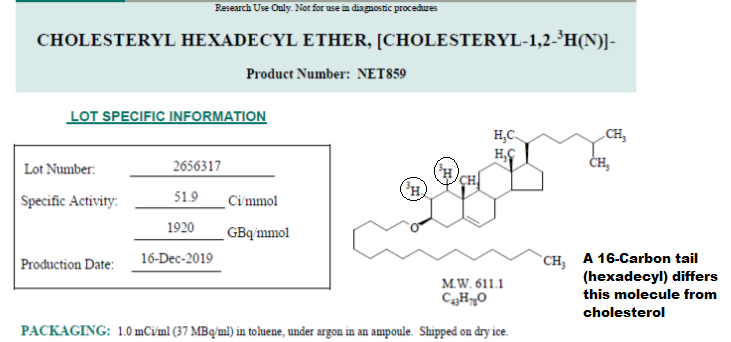
As you can tell from the structure above this radioactive compound looks just like cholesterol. The main difference, aside from the addition of the tritium (circled above) is the 16-carbon hydrophobic tail located at the bottom.
Depending upon the study being conducted researchers may be concerned about metabolism of their deployed compound. In metabolism studies different carbon atoms may be swapped for radioactive isotopes. In these studies isotopes allow for researchers to both figure out what metabolites are formed from their drugs and where they end up in an animal. In distribution studies researchers need to be careful that their compound is not metabolized. Otherwise, they would not be getting an accurate depiction of distribution if researchers cannot figure out if their compound has been metabolized. Essentially they both are studies that deploy radioactive compounds with different intents.
The cholesteryl hexadecyl ether used in this study is both metabolically inert (won’t be metabolized) and cannot undergo exchange with other lipids. This means that 3H-CHE will most likely stay within the LNPs until it reaches a cell and gets uptaken. Afterwards, it is not metabolized and is excreted from the body intact. This is likely due to the addition of the 16-carbon hydrophobic tail that anchors the molecule into the LNPs. It also prevents it from being recognized by enzymes.
All this to say that we can be quite confident that the measured of radiation within this study can be a somewhat accurate representation of where these LNPs have ended up, although we will still examine this information with hesitation.
Introduction
Again, the study was fairly straightforward here. The researchers used Winstar Han rats as their animal model. The LNP formulation used was the LNP8 formulation from the prior study. It’s also the same formulation deployed in the Pfizer/BioNTech vaccines. Inactive luciferase mRNA was used instead of spike mRNA.
First, the researchers wanted to see what level of LNP would be tolerable to the rats so 21 male Winstar rats were provided the equivalent of 100 ug mRNA LNPs. However, the high number of adverse reactions caused the researchers to use a lower dose:
Although the toxicity could be attributed to LNPs the possibility of acute radiation poisoning should not be discounted. Nonetheless, half the dosage was used in these studies (50 ug mRNA LNPs).
A total of 42 rats were used in this study (21 male and 21 female). The rats were then subdivided into 7 different time points with 3 rats per timeframe- this is important to discuss later. Rats were injected with 50 ug mRNA LNPs intramuscularly. At specific time points rats were sacrificed and tissues, organs, and blood/plasma were collected and examined for radioactivity.
Results
Injection Site Results
Looking at the results we’ll start to notice some concerning standout data:
For the males you can see a somewhat consistent increase in radioactivity within the injection site up to hour 4. However, for the female rats the values are far too inconsistent. You can see that hours 1, 2 8, and 24 show the highest level of radioactivity within these animals.
Why are these results so inconsistent? We have to remember that the rats needed to be sacrificed in order to collect their tissues and examine radioactivity. That means that at each time point a different set of triplet rats were sacrificed. Essentially, measurements from different rats were used to compare.
Although this may be a typical methodology for this type of study, the big issue here is that individual differences in pharmacokinetics and other variables mean that each cohort could vary greatly from one another. The distribution of each LNP in each rat may not be consistent, such that mice used in the 2 hour cohort may have a widely different distribution of LNP compared to the rats used in the hour 8 cohort. This is likely why the female data is so inconsistent.
This also creates problems with possible sex-dependent biodistribution pathways. You’ll notice that the researchers indicate that increases within the injection site occurred up to 4 hours post injection, yet this is only possible if the researchers only reference the male rats. It’s made even worse when you realize that one of the the lowest levels of radioactivity measured in female rats occurred at the same timepoint that the male rats had the highest levels.
With such inconsistent data, why was this not addressed by the researchers? Clearly the timed cohorts are difficult to compare, and the differences between the sex cohorts are even more egregious. The idea that such a drastically different value was not addressed is extremely concerning. I would argue this means that many of the other values are difficult to evaluate.
We have to also keep in mind that, unlike luciferase expression which depends upon the translation of luciferase and luciferin breakdown, there’s no real way of hiding radioactivity. That means that there really should be no reason for the radioactive levels within the female rats to have drastically dropped and spiked back up again. Again, the inconsistency should really be called out here.
The researchers do provide a reason why this may have occurred, although this explanation is actually makes me more concerned about the protocols used in this study:
When analysing the injection site, there was often high inter-animal variability in concentration and % injected dose values at each time point. This may have been due to difficulty in collecting the entirety of this sample since the total area that the injected bolus dose migrated to within the muscle was not visible. When dosing the male 50 µg mRNA group, the injection site was circled using a marker pen to help aid dissection of the injection area. The overall injection site concentrations and % dose values were higher in males than in females. Since concentrations in other tissues were broadly similar between the sexes, it is likely that the higher injection site values in males were a result of its more consistent identification and collection in males.
The injection site results were used as a validation that these vaccines did not move, yet the researchers didn’t even bother to accurately collect tissues from the injection site to provide for proper results (they couldn’t even tell where the proper injection site was for females). Again, this explanation actually makes me concerned about the results and how much proper procedures were followed.
Remember to keep this in mind when examining the other data. There’s a lot of issues with these results and the idea that these were not addressed is startling.
Target Organ Results
Next, the researchers compared time-lapsed radioactive data in tissues with the highest levels of radioactivity:
Unlike the results of the injection site the results here are actually consistent, possibly due to more proper selection of the tissues. We can see that, over time, there tends to be an increase in radioactivity within organs such as the liver, spleen adrenal glands, and ovaries. There have been questions raised as to what organs or tissues may be targeted, with some hypotheses attributing the targeted regions to fat distribution because the LNPs themselves are lipid-based.
However, Brian Mowrey of Unglossed provided me an article that actually changed my perspectives on the structure of the LNPs. Again, a big shout out to Brian for providing me the article, and please check out his Substack!
The article comes from Chemical & Engineering News from April of last year. It provides a pretty broad analysis of the history and development of LNPs leading up to the structures used in these vaccines.
But what really stuck out was the explanation it gave for the development of siRNAs and the structure it provided (emphasis mine):
The most effective nanoparticles were ones that the body mistook as low-density lipoprotein (LDL) cholesterol—commonly called bad cholesterol. Proteins that recognize LDL cholesterol in the blood bound to some of Alnylam’s nanoparticles and carried them to LDL receptors on liver cells, which then caused the cells to engulf the nanoparticles in an endosome. It was the kind of complex interplay that studies in a petri dish missed.
This justification is important. For many talks of the LNP I have disregarded them as just a vehicle for the mRNA. I also found no real concerning evidence about the LNPs and toxicity, although I am open for information to come out refuting this claim. What’s important with this information is that it actually provides some perspective as to how our bodies may view these LNPs, such that they may not see them as innocuous blobs of fat but may actually consider them to be indicative of some of the lipid structures within our own body. In this case, we may have to wonder if our bodies are being tricked into thinking that these LNPs are carriers of cholesterol and are shuttling them to certain organs.
Why would this be important to the organs indicated above? Cholesterol serves as a precursor for many of our bodies hormones including estrogen and testosterone. The endocrine system relies on exogenous sources of cholesterol to help produce these hormones. What may be happening, although this is very rudimentary thinking, is that the LNPs may be moved to certain organs because the body is being tricked into thinking it is moving “cholesterol”, and thus is moving the LNPs to areas where cholesterol is needed. This would explain why organs such as the adrenal glands and ovaries are targeted.
However, this does not explain the targeting of the spleen or liver. The liver is involved in lipid metabolism and the spleen is involved with filtering the blood. It could be that accumulation within these organs are due to their roles in metabolism and filtration, with buildup occurring due to the funneling of radioactive 3H-CHE to these organs.
It also does not explain why the testes are not targeted. Differences in androgen production between the sexes may play an important role but that requires analysis of androgen production.
There are also quite a few structural differences between the LNPs described in the article and those used for the mRNA vaccines. For one, the structure and description was based on siRNAs1 (small interfering RNAs) which are much smaller compared to the spike mRNAs. The LNPs used in siRNA formulations are also different than the ones used in spike mRNA formulations.
Taking all of this into account, this idea is more speculative than grounded in actual evidence. However, this should be something that is taken into account. Researchers who may be interested in understanding why the LNPs are migrating to certain organs should understand how are bodies are viewing these LNPs and whether they may be mistaking them for other forms of endogenous lipid structures.
Overall, we know that certain organs are likely to pick up these LNPs. The reason why this is occurring is still left to be determined.
Questionable Lab Practices?
I tend to overlook the materials and methods sections, but it is here that you are likely to see protocols and procedures that stick out. When results are data do not look accurate, try to search the materials and methods section to see if any deviations may have occurred to bring skepticism to the results.
It’s here where I noticed that the collection of injection site tissues was wholly inconsistent, possibly to the point that studies should have been redone (how can you use data for tissue that you cannot even determine that you have collected?).
But this isn’t the only instance worth questioning.
Apparently, the wrong bone samples were collected for 8 of the 21 male rats.

Now, it is stated that this correction was done as soon as the errors were noticed. However, 7 out of 8 of these mice were sacrificed at the earlier timepoints of the study. That means that, unless these errors were caught immediately after tissue collection, these results are likely to be a wrong account for the time period. Essentially, that would suggest that the femur results should be excluded from analysis.
It may seem like this is nitpicky, but remember that experiments are done under heavily controlled environments. At the point that results are likely to heavily be altered due to time, the results should no longer be considered accurate. Usually such an egregious error would lead to a redo of many studies, but the idea that such an error was made and the results were still accepted are quite alarming.
Yes, it is just a femur but this is representative of possible sloppy lab practices that are altering the results that are being presented to the FDA and therefore the results being used to validate the so-called safety of these vaccines.
It reminds me of the Pfizer whistleblower who stated that data collection during clinical trials were not done appropriately and that proper practices were not being followed. Such a revelation should have made us assume that we should be on the lookout for more of these poor practices occurring, and here we can see a clear example that egregiously sloppy lab practices are occurring.
Remember, how could it be that BioNTech could report that most of the LNPs stayed within the injection site when they did not even properly collect samples from the female rats? Add in the fact that these LNPs may be aggregating within the ovaries of the female rats and it raises even more cause for concern about how many actual LNPs are staying within the injection site of female rats.
These results helped to validate the use of these vaccines in millions of people, and if Pfizer and BioNTech could not bother to follow proper lab procedures or to redo studies how do we trust that these vaccines are safe and effective without any proper studies?
Conclusions and Takeaways
The rest of the results are unremarkable, essentially showing small amounts of LNPs distributing to other parts listed above. What’s interesting is that LNP accumulation did not appear to have occurred in the hearts of male rats. Longer study periods may provide different information, but it raises questions as to the role LNPs play in myocarditis and if mRNA delivery may be the cause.
We may also have to keep in mind that, even though I have described the results within the context of LNPs, we have to remember that only the cholesterol analogues were radiolabeled. Therefore, we have to keep in mind that it is in fact the 3H-CHE that is being traced and not the entire LNPs themselves. This should raise skepticism as to how closely the 3H-CHE compares to actual LNP distribution.
Even more concerning is the type of lab practices that are being accepted with these studies. We have clearly seen that there were many deviations (aka poor practices) that are likely to have changed the results of these studies. Considering that there is evidence of poor practices occurring with the clinical trials already we need to be aware of how many of these poor practices may come about in other studies. This should also raise questions as to how these results could have been accepted and approved.
To conclude, here are a few more takeaways:
The reported injection site results seem to stem mostly from the results of the male rats. Poor tissue collection of female rats, along with the concerningly high levels of radioactivity within rats’ ovaries, means that there’s no actual proper measure taken for females. That means that there’s no way of knowing how much of the LNPs may have stayed within the injection site. Possible sex differences in pharmacokinetics needs to be addressed.
The Liver, Spleen, Adrenal Glands, and Ovaries showed the highest levels of radioactivity. Why these organs were targeted are still questionable. It could be because of excretion of the radiolabeled 3H-CHE, or it could be movement of lipids through the body. However, the C&E News article suggesting LDL cholesterol mimicry may allude to another possible pathway. If these LNPs are being recognized as LDL cholesterol and are being distributed accordingly, it may explain accumulation within the adrenal glands and ovaries. Research into the roles of these LNPs should look at LNPs from the perspective of how our bodies may be perceiving them.
Questions should be raised about poor laboratory practices and data collection. Just like what was alluded to by the Pfizer whistleblower, this study contained several concerning evidences of poor laboratory practices. How much this affected the results should be brought into question. This should also raise concerns as to how many other laboratory practices may have been compromised. As more documents are released people should remain alert for other evidences of poor laboratory practices.
Taking both this study and the prior study (R-20-0072) into account, we can see that there is a lot of travelling of the LNPs occurring. Consilience tells us that not only are these LNPs accumulating within the liver, they actively translated luciferase which showed activity within the livers of mice. Taken together, this suggests that the LNPs may be travelling and are bioactive. Unfortunately, the evidence so far only points to this occurring within the liver. Whether spike proteins may be produced within the ovaries or adrenal glands of vaccinated individuals is yet to be determined. However, these studies certainly suggest that the idea that these LNPs are not travelling have been thoroughly disproven.









Wasn’t there an earlier Japanese study finding similar concentrations of LNPs in those organs? As soon as I saw that the ovaries were collecting that stuff the shot was a big NOPE for my 12-year-old daughter. It still horrifies me that just about all her friends got the shots, no questions asked by their parents. Well, that all healthy kids got it, really, but that’s another rant 😜
"I tend to overlook the materials and methods sections"
Haha, that's where I usually head to first. I don't wait until the results appear suspicious, I assume all study results are suspicious to begin with! And yet ironically, I find the authors' rationale here to be compelling. Accurate sampling for non-injection-site organs was easier than for the injection site, and so the wild variations in injection-site values are just darts that missed the bullseye and should not necessarily condemn the non-injection-site results, which are a lot more consistent for most organs except the hearts (Table 3). In a sense, there was no point attempting to quantify the injection site to begin with. The variations are extreme even at .25 hours (appendix). It's a lot harder to sample "area in the leg that was hit by a needle" than "bladder" since one can "move" and the other can't.
A really great walk-through (I especially got value from the examination of whether distribution can be measured with fidelity by this type of experiment to begin with), and thanks again for the kind link. Not only should this have prompted rescinding the EUA application, it's sickening to think that the results here didn't even wrap until Phase II/III was well underway.
Obviously, your cholesterol-transport mechanism, if correct, would still potentially be competing with capillary fenestration for determination of uptake, and this could account for both glandular and liver-targeting. See figure 5 of https://www.nature.com/articles/nrd4278 (which was cited by Pfizer in their FDA application!).