Largest COVID vaccine study published to date recognizes serious adverse events
A study encapsulating nearly 100 million people note higher than expected reports of GBS and cardiovascular events post-vaccination.
Edit: Grammatical correction was made below (Not was added to the beginning of the malfeasance sentence). Thanks to nobler for pointing that out!
Last week a study was published in the journal Vaccine1 examining adverse events associated with COVID vaccines, taking a look from a global perspective.
The timing seems to coincide with the widespread reports of Good Ol’ Joe’s escapades in being “cognitively unfit”, possibly drawing attention away from this study- just a random thought I had and not pointing to any conspiracy.
This study is the largest of its kind, encapsulating almost 100 million individuals, over 180 million vaccination events, and spans over 8 countries included in the Global COVID Vaccine Safety (GCoVS) Project, which is part of the Global Vaccine Data Network™ put in place by the CDC to monitory vaccine safety data from across the world.
The countries included in this study include:
Argentina
Australia
New South Wales
Victoria
Canada
British Columbia
Ontario
Denmark
Finland
France
New Zealand
Scotland
Surprisingly, America is not included even though this seems to be part of an initiative put forth by the CDC.
The study period spans from December 2020 up until August 2023. Bear in mind that data collection was not consistent among the included countries, with some countries only comprising a little less than a year of data as in the case of New South Wales, Australia. Argentina seems to have the longest data collecting time period of 32 months, and likely serves as the time frame mentioned between 2020 and 2023.
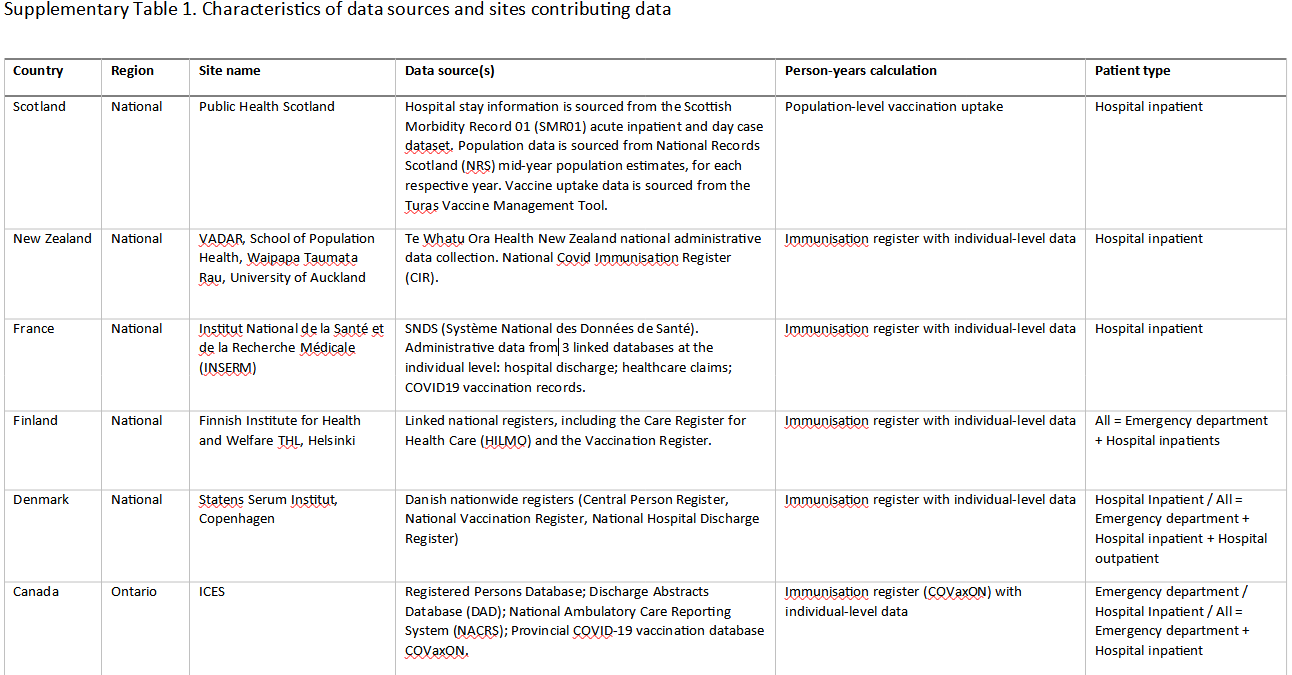
Also, note that data collection was heterogeneous, with patient type recorded being different across countries where some may have calculated solely on inpatient hospitalization while others may have included both emergency visits and inpatients.
Take note of some of these differences in reporting from a portion of Supplementary Table 1:
Breakdown between sex, age, and dosage across the countries can be noted in Table 1 (if difficult to read look at the table through the study- note that there seems to be a misprint in the Table as Moderna’s vaccine is shown twice and ChAdOX1 is missing):
Surveillance data was limited to within the first 42 days after receiving a dose, with the risk breakdown being separated by the following time periods:
0-7 days
8-21 days
22-42 days
0-42 days
Like with other studies Day 0 refers to the date of vaccination.
The adverse events that were examined were limited to a list of Adverse Events of Special Interest (AESI), so keep in mind that not all adverse events were examined and therefore some information is being missed with this dataset. The list of AESI can be seen below from Supplementary Table 2:
Odds ratio of adverse events were calculated based on pre-COVID vaccine data, serving as a baseline for expected event rates:
For each site, we calculated the observed number of events for each AESI in the risk interval after introduction of COVID-19 vaccination. To calculate the expected number of cases, we used pre-COVID-19 vaccination background rates data from 2015 to 2019 (2019–2020 for Denmark) collected in the GCoVS Background Rates of AESI Following COVID-19 vaccination study [13]. The observed follow-up period in person-years for a given vaccination profile and post-vaccination period was stratified according to age group and sex. Each of the age-sex stratified person-years were multiplied by the corresponding age-sex stratified background rate. This resulted in the expected number of cases in each stratum, which were then summed to give the total number of expected cases during the observed follow-up period.
In summary, when the data was examined there was a statistically significant safety signal regarding the adenoviral-vector vaccines and GBS rates, as well as CVST. Note that the following colors notes statistical significance, with yellow and red noting statistically significant odds ratios regarding adverse events and the COVID vaccines listed.
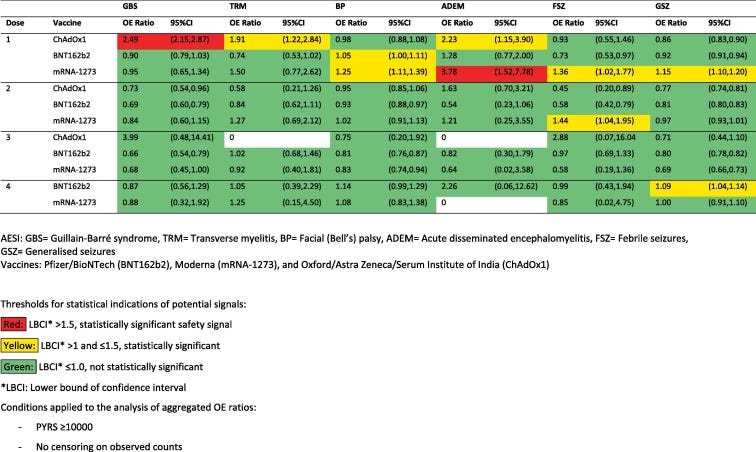
And when it comes to cardiovascular issues note that all 3 vaccines were noted to have statistically significant odds ratios, with the mRNA vaccines showing statistically significant safety signals:
There are some interesting things to point out here. Note that neurological and hematological AESI are noted predominately with the 1 dose. There are several factors to consider here, as adverse events may make people less inclined to get further doses and therefore may select for a bias in favor of the perception that further doses appear safer. It’s important to keep in mind that the population demographics in question are likely changing between doses.
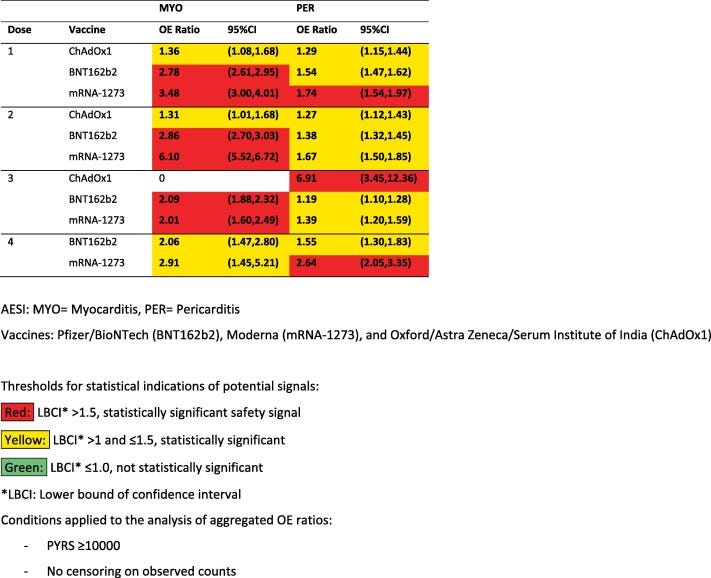
However, what’s interesting is that cardiovascular-related AESI are increased across all doses, and thus raises a question about the differences in how adverse events are manifesting. The argument over adverse event dropouts wouldn’t explain why people may experience cardiovascular-related AESI at dose 3 or 4. One possible explanation may be the heterogeneity of symptom onset, where people who may have unnoticed cardiovascular damage after the first or second dose may experience symptoms after receiving boosters, leading to late dropout from repeat vaccination.
Bear in mind that the adenoviral-based vaccine ChAdOX1 shows a very high odds ratio of near 7 for pericarditis during dose 3. One question I would ask is whether this may be a shuffling effect where people who may have gotten an adverse event from the mRNA vaccines may have been persuaded to go with a different vaccine platform rather than foregoing them entirely. There may be a bias to the extent that people who would have dropped out from mRNA boosters may be shuffled into the ChadOX1 group leading to the very high safety signal noted. The population receiving the ChAdOX1 booster are likely to be far lower as well, meaning that any noted AESI may bias higher.
For those interested take a closer look at the study itself as this is intended to be a quick view.
What’s important is that this study corroborate many of the anecdotes and studies that have been in circulation for years, only this study helps to emphasize the concerns over these vaccines on a global scale. It raises questions regarding the extent of possible adverse events being seen worldwide, and emphasizes the need for greater surveillance and more diligence in providing answers to how damaging these vaccines could be.
It also raises questions over why certain AESI manifest at different dosages.
Bear in mind that even with such a large population study there are still so many things that are left unanswered, including the susceptibility of certain demographics (i.e. do certain adverse events manifest more commonly among certain demographics), as well as the number of other adverse events that were not included in this study.
And what’s likely to be more important is understanding what all of this may mean in the end.
To that point, the authors end their study with the following disclaimer, making a point to note that the results of this study don’t reflect the views of the CDC:
All analyses, inferences drawn, opinions, conclusions, and statements are those of the authors and do not necessarily represent the official views of, nor an endorsement by, CDC/HHS, or the U.S. Government. For more information, please visit cdc.gov.
Parts of this material are based on data and/or information compiled and provided by the Canadian Institute for Health Information and the Ontario Ministry of Health. The analyses, conclusions, opinions, and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred. Parts of this material are based data and/or information provided by the British Columbia Ministry of Health. All inferences, opinions, and conclusions drawn in this manuscript are those of the authors, and do not reflect the opinions or policies of the Data Steward(s).
But then what exactly is the stance of the CDC, and to what degree of seriousness are they taking the VAERS reports and anecdotes that continue to come out? What does it mean when a study based on data provided to the CDC runs counter to the narrative the CDC itself puts out regarding these vaccines?
The CDC needs to address these findings. Not doing so would be considered malfeasance and an act of neglect. The fact that mainstream outlets such as Bloomberg is covering this study points out that this isn’t something that the CDC can just obfuscate.
If anything, studies such as these help to put the CDC in the spotlight of accountability.
If you enjoyed this post and other works please consider supporting me through a paid Substack subscription or through my Ko-fi. Any bit helps, and it encourages independent creators and journalists such as myself to provide work outside of the mainstream narrative.

Faksova, K., Walsh, D., Jiang, Y., Griffin, J., Phillips, A., Gentile, A., Kwong, J. C., Macartney, K., Naus, M., Grange, Z., Escolano, S., Sepulveda, G., Shetty, A., Pillsbury, A., Sullivan, C., Naveed, Z., Janjua, N. Z., Giglio, N., Perälä, J., Nasreen, S., … Hviid, A. (2024). COVID-19 vaccines and adverse events of special interest: A multinational Global Vaccine Data Network (GVDN) cohort study of 99 million vaccinated individuals. Vaccine, S0264-410X(24)00127-0. Advance online publication. https://doi.org/10.1016/j.vaccine.2024.01.100






That is all that is left, accountability, the damage whatever it is now and in the future has been done. What a completely reckless endeavour.
Correction: "Doing so would be considered malfeasance.." should be "Not doing so would be considered malfeasance.."