This is further discussions into vaccines that extend upon Friday’s leaky vaccine discussion. Part I of this vaccinology talk can be found here. Please note that these reviews are not all-encapsulating and will miss many key points in the vaccine discussion.
Note: Additional images and figures have been added.
All that’s vaccinated is not immunized
Normally, I would have never considered to what extent any vaccine I got worked. I used to get annual influenza vaccines and took it as a one and done. I got it, I’m fine now, right?
Well, you may be surprised to find out that, depending on the vaccine, up to 10% of vaccine recipients never mount a proper immune response after vaccination (these people are referred to as non-responders).
Vaccines themselves are a highly intricate system of structures and biochemical processes. Immunology even more complex- scientists haven’t fully elucidated the entire mechanisms involved in our own immunity. Pair them together and it’s a recipe for examining highly complex systems.
Vaccines are not necessarily a one and done deal. There’s many factors one must consider when examining whether a vaccine really will work.
Unfortunately, sometimes they don’t, and vaccine recipients may experience a phenomenon called vaccine failure.
There’s a few definitions for vaccine failure, and they’ll mostly depend on what we expect from a supposedly “perfect” vaccine.
Again, do we expect a vaccine to neutralize the virus, should the recipient be able to transmit the virus to others, or should the recipient be protected from falling ill?
User TheGreatAwakening provided a list that appears to have come from Dr. Peter McCullough and his idea of what constitutes a proper vaccine:
Must give you antibody immunity to virus or bacterium
Antibody must give protection from the virus or bacterium
Injection must show it reduces hospitalizations, deaths, or severe symptoms of the virus or bacterium
Stops you from carrying the virus or bacterium (infectiousness)
Stops the transmission of that virus or bacterium from one person to the next
From this, we may assume that a perfect vaccine has all of the traits listed above. Keep in mind that this list didn’t come directly from Dr. McCullough himself and is second-hand, and so be careful in directly attributing these points to him.
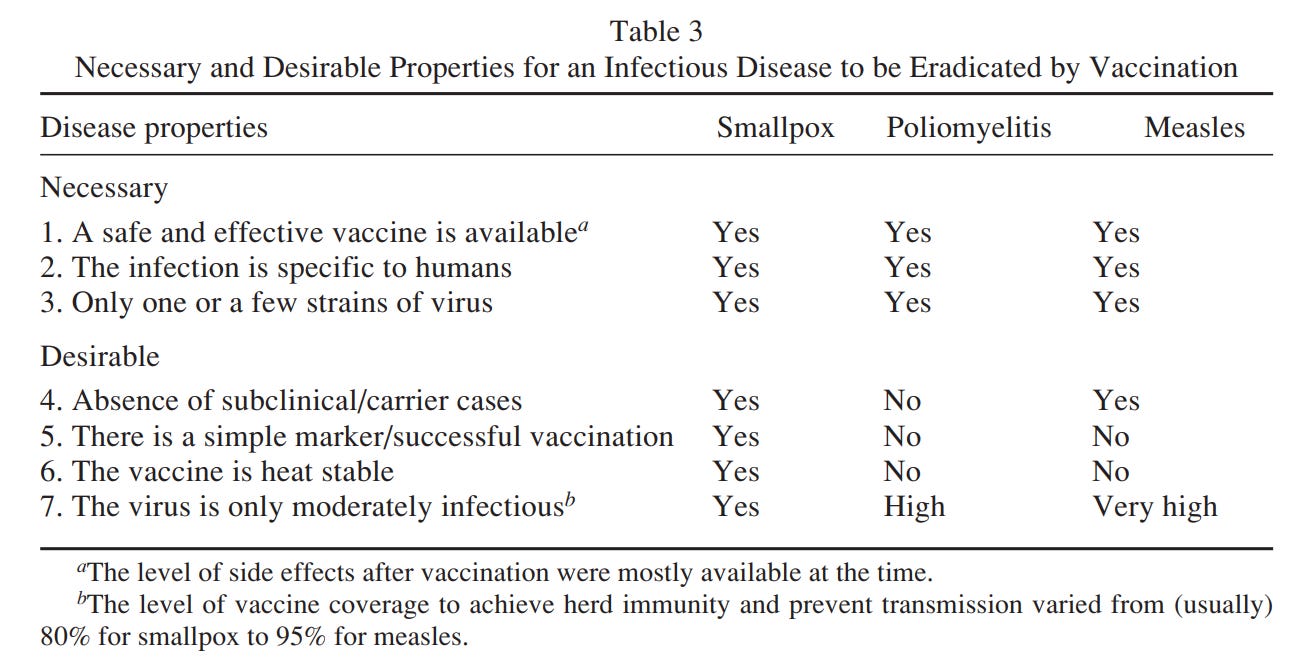
Ada, G.1 provides a table describing what traits are both necessary and desirable if a vaccine is to eradicate a disease, with a few examples listed:
The list above points to an interesting feature of vaccines- if any animal reservoirs exist, if the virus can still be transmitted, and an infected individual is still infectious then this sort of vaccine may be considered undesirable (at least to the extent one would want a sterilizing vaccine).
And so, in describing what constitutes vaccine failure, it may be appropriate to consider what an ideal vaccine is, with the assumption that vaccine failure will be a deviation from said ideal vaccine.
That means a vaccine that fails is likely to break the rules put forth above2- vaccine failure suggests that the vaccine may not provide a proper immune response to deal with the intended pathogen, it may not stop the recipient from becoming infected and symptomatic, the recipient may still transmit the pathogen to others, and so on and so forth.
But again, what leads to vaccine failures? Why does administering a vaccine to someone not provide them property immunity?
Types of vaccine failures
On Friday I didn’t properly set up the framework to discuss a “leaky” vaccine. So here I will briefly revisit the discussion and use the context from this article.
As I stated on Friday’s post, most measures of vaccine effectiveness are derived from technical, mathematical modes of modeling vaccination. This makes it rather difficult to put it into layperson terms. More importantly, vaccine effectiveness is not necessarily measured based on a vaccine’s actual ability to reduce infection, transmission, or lead to herd immunity but instead is based on a vaccine’s inability to do so- essentially a vaccine’s failures.
And it’s here where the terms used on Friday are derived. If we start with the idea of what an ideal vaccine is, we can then categorize vaccines based on their failures. Below I will focus on two, but keep in mind that there are different categorizations out there, such as a waning vaccine.
“All-or-Nothing” Vaccines
AoN vaccines are the closest thing to an ideal vaccine. It is one in which vaccine recipients are fully protected against the target pathogen. The main caveat of AoN vaccines is the subset of non-responsive vaccine recipients who do not receive proper protection. For example, such a scenario may be one in which 100 people receive a vaccine; 90 will receive full protection while 10 may be non-responsive and still be susceptible to infection. Such a vaccine would be considered to have a “vaccine effectiveness” of 90%, with the caveat that effectiveness is being used broadly here.
This feature is considered a vaccine failure in “take” since some vaccines will not take to those who they were provided. AoN vaccines act independent of pathogen exposure, meaning that they should provide protection in all scenarios. Again, AoN is the closest thing to an ideal vaccine. The measle vaccine is an example of an AoN vaccine.
“Leaky” Vaccines
Although there are many caveats in defining a leaky vaccine, leaky vaccines essentially exist on the opposite end of the vaccine spectrum (opposite that of AoN vaccines). Their name likely derives from the fact that the virus or bacteria may “leak” through a person’s immune protection. They generally break many of the rules outlined above, with the inability to provide sterilizing immunity being one of the biggest failures of leaky vaccines. Because of this, leaky vaccines are considered to suffer from failure in “degree”. Since they do not provide full protection, their protection is heavily contingent upon the relationship between the pathogen, the vaccine, and the vaccine recipient.
More importantly, leaky vaccines are heavily exposure-dependent. The more one is exposed (or is at risk of exposure) to a pathogen the greater the odds that the vaccine will not hold up. Viral load, viral transmissibility, and degree of circulating virus among a given population can greatly alter how “effective” a leaky vaccine is. Malaria, influenza, and SARS-COV2 vaccines can be classified as leaky vaccines.
Keep in mind that several liberties have been taken in defining a leaky vaccine. Some definitions describe leaky vaccines as one in which each exposure carries an equal risk of infection for everyone (Crowcroft, N. S. & Klein, N. P.), but I personally don’t consider this to be an adequate definition and is itself rather vague.
In general, AoN vaccines are ideal, and any vaccine that deviates from an AoN vaccine may be considered a leaky vaccine.
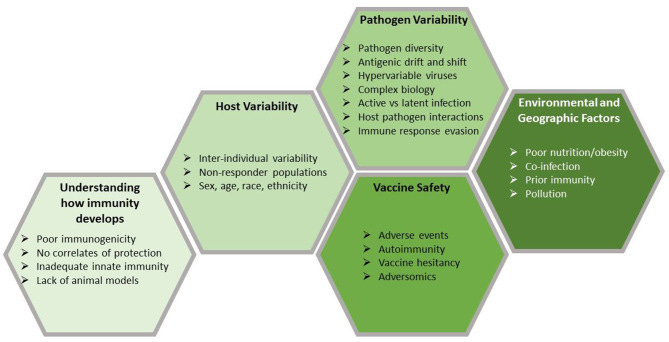
The intersection of host, vaccine, and pathogen
Here’s some food for thought: we know full-well that obese individuals are far more likely to suffer from severe COVID. We also know that improper immune function will lead to severe COVID as well. Therefore, may we assume that obese individuals will, in some form, be immunocompromised, and thus not take well to a vaccine that requires a properly functioning immune system (immunocompetent).
Many modern vaccines don’t involve infecting a recipient with the pathogen, but there is still an expectation that the immune system recognizes and mounts a response to something that doesn’t look right.
But the immune system is also an extension of the body’s overall processes- it’s just one piece to the puzzle that is an individual’s general health. As such, we should expect that our immune system’s protective capabilities rely on our body being in a well state.
Indeed, not only is an effective vaccine as good as it’s ability to induce an immune response, but relies heavily on the variability between vaccine recipients and their own individual health and behaviors.
From Wiedermann, et. al.3:
There are 2 major factors responsible for vaccine failures, the first is vaccine-related such as failures in vaccine attenuation, vaccination regimes or administration. The other is host-related, of which host genetics, immune status, age, health or nutritional status can be associated with primary or secondary vaccine failures. The first describes the inability to respond to primary vaccination, the latter is characterized by a loss of protection after initial effectiveness.
And so variability between both the vaccine and the recipient play into whether a vaccine “fails”.
Vaccine-related Failures
Several variables are involved in vaccination, all of which may alter the body’s immune response and ability to protect against the desired pathogen. Most notably, the vaccine formulation in use for a specific pathogen (such as SARS-COV2) may confer different types of immunity. One underlooked feature includes the route of vaccination, which also is likely to contribute to vaccine failure.
Vaccine formulations
Different vaccine platforms may elicit different immune responses, with some of them not being of high fidelity.
Let’s take the COVID vaccines, for one. So far there have been 4 different vaccine formulations (mRNA, viral-vector, antigenic, and inactivated virus) each with different relative risk reductions. We’ll correlate relative risk reductions to “vaccine effectiveness”, even though that’s not an entirely proper assumption to make. But considering that the clinical trials should each have a similar methodology, we’ll make an argument in favor of consistency rather than accuracy.
Here’s a list of the RRR for these different formulations (taken from the first Phase III clinical trials):
Note that J&J’s vaccine is the only initial single-dose vaccine listed above. Nonetheless, based on the RRR data alone we can see that different vaccine formulations may elicit different immune responses. This can come down to many different factors such as dosing, antigen presentation, types of antibodies produced, etc.
Once again, keep in mind that the RRR taken from the clinical trials does not indicate what immunological processes are occurring, but provides a general picture as to even a difference in vaccine formulation can alter a vaccine’s supposed effectiveness.
Overall, it at least suggests a relationship between the type of vaccine given and the type of protection one is assumed to receive.
Vaccination Site
Have you ever wondered why the annual flu vaccine is usually administered in the shoulder when the virus enters into our bodies primarily through our mouth and nose? Have you ever considered if this would affect how well your body deals with the virus?
Now, we won’t go into immunological detail here, but the key player in antibody production is our lymphatic system. Our lymphatic system is a collection of organs, tissues, and vessels that shuttle both toxins and antibodies around our bodies (among other agents).
What’s important to this discussion is evidence that our lymphatic system is partly compartmentalized. Most notably, there’s a distinct difference in immune function between our upper airways (usually categorized under mucosal immunity since these regions are covered in mucus membranes and produce different immunoglobulin antibodies) and the rest of our body (referred to as systemic immunity). Essentially, this means that the vaccines we get in our shoulders may not produce a proper protective response in our upper airways, which certainly wouldn’t help fight against upper-respiratory infections such as the cold or flu.
A more technical explanation can be seen below in regards to SARS-COV2 immunization (Russell, et. al.9, emphasis mine):
Almost all efforts at vaccine development against COVID-19 focus on systemic injection, which predominantly induces circulatory IgG antibodies and, potentially, cytotoxic T cells (18). These routes are poorly effective at generating mucosal immune responses, which can only be induced by mucosal routes of immunization, including through the NALT in the URT. Mucosal immune responses are partly compartmentalized, as the distribution of the responses depends on the actual route of induction (7, 19). For example, the enteric route predominantly generates responses in the gastro-intestinal tract, whereas the nasal route predominantly generates responses in the respiratory tract and salivary glands (7). The reasons for these differential distributions lie in the imprinting of the T and B cells induced in the respective inductive sites, the gut-associated lymphoid tissues (GALT [gut-associated lymphoid tissue], such as the intestinal Peyer’s patches) or NALT [nasopharynx-associated lymphoid tissue], with “homing” receptors including specific integrins and chemokine receptors specific for the target tissues (20). In practical terms this means that intranasal immunization should be an effective means of generating predominantly SIgA antibody responses in the URT [upper-respiratory tract] and LRT [lower-respiratory tract], where SARS-CoV-2 could be neutralized and eliminated without inflammatory consequences. In addition, it implies that assaying IgA antibodies in nasal secretions or saliva should be a more informative way of assessing effective immune responses against SARS-CoV-2, whether induced by the natural infection or by intranasal immunization.
This argument tends to be overlooked in many analyses of vaccine effectiveness. Where a vaccine is administered could greatly determine how protective a vaccine is, and an improper route may lead to vaccine failure.
Host-related failures
Once again, a person’s immune system is only as good as the overall health profile of the individual. In instances where someone’s health may begin to falter, so too may that person’s immune system. Thus, by inference, a faulty immune system should not be able to mount a proper protective response against both a vaccine or a pathogen.
There’s a lot of factors that make up an individual’s immune profile, such as their age, sex, race, family history, dietary habits, and lifestyle. For the sake of simplicity, we will stick to an example of age and a comorbidity (obesity) as it relates to immune function, and thus vaccine failure.
Age and Immunosenescence
An aging body becomes more frail, and so too does one’s immune system. The act of aging is called senescence, and in biological terms it refers to our cell’s nearing the end of their replicating capabilities. Yes, our cells have a limit to how many times they can replicate and divide (referred to as the Hayflick-limit), and at the point that they can no longer divide an individual grows even slightly closer to death. A rather morbid state of affairs!
A similar phenomenon occurs with our own immune system, and this phenomenon has been termed another portmanteau immunosenescence (aging immune system).
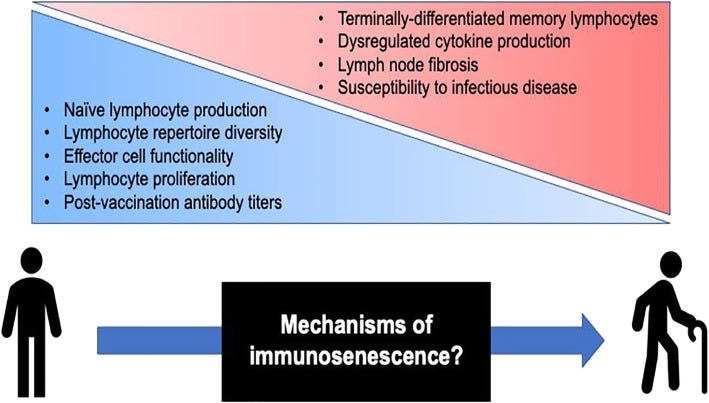
Immunosenescence has been implicated in a host of diseases (Crooke, et. al.10):
Aging is associated with the decline of various biological systems and the development of numerous co-morbidities, such as diabetes mellitus, cancer, and various autoimmune and neurological disorders [1]. The immune system suffers equally from the effects of biological aging, exhibiting a progressive decline in function—referred to as immunosenescence—that collectively results in diminished humoral and cellular immune responses [2–4]. (Figure (Figure1)1) Mechanistic analyses of immunosenescence are complicated by the integrated nature of the immune system, as it is difficult to discern if immune cell dysregulation is a result of inherent cellular changes or a reactionary mechanism to changes elsewhere in the body. Regardless of origin, functional differences in the aging immune system are well-documented [5, 6], and several studies have attributed negative clinical outcomes in populations of older adults (i.e., 65 years and older) to immunosenescence.
Albeit rather complicated, a lot of these issues are derived from a dysregulated immune system which may no longer be able to keep diseases such as cancer and prion formation in check. In cases of new infection, the naïve B and T-cell repertoire of an elderly person means that they may not be able to mount an adequate response to pathogens.
Unfortunately, this deterioration in immune quality also carries over into vaccination, and is likely a key player in vaccine failure among the elderly (emphasis mine):
The success of vaccination among older adults is decidedly limited, and adaptive immunosenescence has been implicated as a key determinant by numerous studies investigating vaccine responses in aging populations. Diminished antibody titers have been observed among older adults [16, 17], and, in many cases, the quality of these antibody responses is markedly inferior compared to those in their younger counterparts [18–20]. It should be noted that declining antibody quality is not a universal age-associated phenomenon, as studies evaluating influenza and tick-borne encephalitis virus vaccines have found no differences in antigen-specific antibody affinity or avidity between age groups [21, 22]. The T cell compartment is also affected during aging, exhibiting contraction of the naïve T cell repertoire [23] and accumulation of terminally differentiated cell subsets with altered effector functions [24, 25]. Additionally, an underappreciated restructuring of lymph node architecture occurs with aging [26, 27], which could further alter critical processes in the development of adaptive immune responses.
In general, we can see that a failure in adequate immune response affects both the ability for an individual to protect against new infections, as well as mount a proper immune response when vaccinated. For the elderly, booster shots have become routine in an attempt to elicit some form of immunity. But in most cases the juice may not be worth the squeeze, and many attempts to produce a protective response may not bear any fruit.
Comorbidities (i.e. Obesity)
Recent scientific breakthroughs have implicated the nefarious nature of chronic inflammation in a host of diseases. Ironically, modern behaviors and lifestyles greatly contribute to chronic inflammation as well.
Considering that the immune system is heavily involved and activated during inflammation, we may assume that chronic inflammation may lead to an impaired, dysfunctional immune system which, again, would affect vaccination.
Additional context is provided in the following excerpt from Wiedermann, et. al.:
Apart from the known secondary diseases related to being overweight, such as cardiac disease, type II diabetes, certain types of cancer or osteoarthritis, recent studies have shown that obesity causes a state of low-grade inflammation, and has direct effects on the immune system leading to immunosuppression.36,37 Accordingly, obese people have been shown to suffer from an increased susceptibility to several infections, such as influenza, tuberculosis or pneumococcal diseases. The mechanisms underlying these immunosuppressive effects are not fully understood but seem to be triggered by the white fat tissue constituting the major part of fat tissues, which acts as an active endocrine organ producing different cytokines and adipokines (e.g. leptins) and thereby interacting with respective receptors expressed on T and B cells.38 In light of the burden of obesity to infectious diseases an adequate protection by vaccination is of particular importance. Generally very few vaccination studies exist in obese people, but it has been documented for some vaccines that an elevated body mass index (BMI) is associated with poor vaccine responsiveness. A study in health care workers having received hepatitis B vaccination showed that the non-responder rate of people with a BMI > 25 was significantly higher compared to persons with a BMI <25.39 Similar findings were recently surveyed in women vaccinated against hepatitis B,37 as well as in a cohort of travelers vaccinated against hepatitis A.2 The negative impact of obesity on vaccine immune outcomes has also been shown for seasonal influenza vaccines40 and for tetanus toxoid vaccine in children.41
In some ways this should come as no surprise. Instead, it greatly emphasizes the importance of our immune system in dealing with all sorts of diseases, including those we may incur on ourselves. Obesity is just one example of a disease in which chronic inflammation may alter immune function, which may both affect infection as well as the ability to respond to a vaccine.
There’s a lot we haven’t discussed here, but remember that a vaccine is not just something you take and become protected. Vaccination relies on a multitude of interactions between the recipient, the vaccine, as well as the pathogen which one should be protected from. This interplay highlights the importance in considering the nuance and subtleties involved, which tends to get missed when discussing vaccines.
Hopefully this outline provides a general understanding of vaccine failures and provides additional context to Friday’s post.
Ada G. (2005). Overview of vaccines and vaccination. Molecular biotechnology, 29(3), 255–272. https://doi.org/10.1385/MB:29:3:255
This definition can be boiled down to a definition provided by Crowcroft, N. S. & Klein, N. P.:
“Vaccine failure is the occurrence of infection or disease in an individual who is fully vaccinated”.
Crowcroft, N. S., & Klein, N. P. (2018). A framework for research on vaccine effectiveness. Vaccine, 36(48), 7286–7293. https://doi.org/10.1016/j.vaccine.2018.04.016
Wiedermann, U., Garner-Spitzer, E., & Wagner, A. (2016). Primary vaccine failure to routine vaccines: Why and what to do?. Human vaccines & immunotherapeutics, 12(1), 239–243. https://doi.org/10.1080/21645515.2015.1093263
Polack, F. P., Thomas, S. J., Kitchin, N., Absalon, J., Gurtman, A., Lockhart, S., Perez, J. L., Pérez Marc, G., Moreira, E. D., Zerbini, C., Bailey, R., Swanson, K. A., Roychoudhury, S., Koury, K., Li, P., Kalina, W. V., Cooper, D., Frenck, R. W., Jr, Hammitt, L. L., Türeci, Ö., … C4591001 Clinical Trial Group (2020). Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. The New England journal of medicine, 383(27), 2603–2615. https://doi.org/10.1056/NEJMoa2034577
Baden, L. R., El Sahly, H. M., Essink, B., Kotloff, K., Frey, S., Novak, R., Diemert, D., Spector, S. A., Rouphael, N., Creech, C. B., McGettigan, J., Khetan, S., Segall, N., Solis, J., Brosz, A., Fierro, C., Schwartz, H., Neuzil, K., Corey, L., Gilbert, P., … COVE Study Group (2021). Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. The New England journal of medicine, 384(5), 403–416. https://doi.org/10.1056/NEJMoa2035389
Falsey, A. R., Sobieszczyk, M. E., Hirsch, I., Sproule, S., Robb, M. L., Corey, L., Neuzil, K. M., Hahn, W., Hunt, J., Mulligan, M. J., McEvoy, C., DeJesus, E., Hassman, M., Little, S. J., Pahud, B. A., Durbin, A., Pickrell, P., Daar, E. S., Bush, L., Solis, J., … AstraZeneca AZD1222 Clinical Study Group (2021). Phase 3 Safety and Efficacy of AZD1222 (ChAdOx1 nCoV-19) Covid-19 Vaccine. The New England journal of medicine, 385(25), 2348–2360. https://doi.org/10.1056/NEJMoa2105290
Sadoff, J., Gray, G., Vandebosch, A., Cárdenas, V., Shukarev, G., Grinsztejn, B., Goepfert, P. A., Truyers, C., Fennema, H., Spiessens, B., Offergeld, K., Scheper, G., Taylor, K. L., Robb, M. L., Treanor, J., Barouch, D. H., Stoddard, J., Ryser, M. F., Marovich, M. A., Neuzil, K. M., … ENSEMBLE Study Group (2021). Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. The New England journal of medicine, 384(23), 2187–2201. https://doi.org/10.1056/NEJMoa2101544
Heath, P. T., Galiza, E. P., Baxter, D. N., Boffito, M., Browne, D., Burns, F., Chadwick, D. R., Clark, R., Cosgrove, C., Galloway, J., Goodman, A. L., Heer, A., Higham, A., Iyengar, S., Jamal, A., Jeanes, C., Kalra, P. A., Kyriakidou, C., McAuley, D. F., Meyrick, A., … 2019nCoV-302 Study Group (2021). Safety and Efficacy of NVX-CoV2373 Covid-19 Vaccine. The New England journal of medicine, 385(13), 1172–1183. https://doi.org/10.1056/NEJMoa2107659
Russell, M. W., Moldoveanu, Z., Ogra, P. L., & Mestecky, J. (2020). Mucosal Immunity in COVID-19: A Neglected but Critical Aspect of SARS-CoV-2 Infection. Frontiers in immunology, 11, 611337. https://doi.org/10.3389/fimmu.2020.611337
Crooke, S. N., Ovsyannikova, I. G., Poland, G. A., & Kennedy, R. B. (2019). Immunosenescence and human vaccine immune responses. Immunity & ageing : I & A, 16, 25. https://doi.org/10.1186/s12979-019-0164-9





For anyone who is curious, the list above that Modern Discontent references from me did come from a video I watched of Dr. McCullough in which he did say all of those five criteria. I paused the video and wrote them all down. Since then, I've not called the mRNA/Adenovirus Vector gene therapies "vaccines".
Can someone tell me if they have difficulties finding my recent posts in their Inbox? I can't seem to find any of my most recent posts, so I'm not sure if Substack has changed the UI to remove posts from the publisher or if my posts themselves are not making it into people's Inboxes. More frustration from Substack...