Hydrangeas and the complexity of Blue
On how a popular mainstay in gardens gets an otherwise uncommon color.
Around this time, you may welcomed with plenty of hydrangeas as they bloom amid the growingly hot days of summer (or at least June). The neighborhoods surrounding my job seem to be hydrangea paradise as nearly every house and business seems to have a hydrangea plant on display.
Hydrangeas (or at least those of the species Hydrangea macrophylla) are a very common ornamental plant not only due to being a perennial and thus easier to maintain, but also due to their large sepals that exhibit vibrant arrays of pinks, blues, whites, and red, making them a very popular choice for gardeners. It also makes for fun walks when trying to spot what color hydrangeas someone may have chilling around their yards.

If readers remember from the blueberry post the color blue is rather rare in nature. Most organism don’t produce pigments that reflect blue light but instead use manipulative tactics to alter wavelengths so that they appear blue.
Your blueberries aren't really blue.
The following is a minor study, and for many it may not be seen as much of a big deal. However, the findings are still rather interesting as it takes something that we don’t really think about and re…
Surprisingly, the original color of hydrangeas are reported to be blue. It’s through modern breeding methods hydrangeas can be found with red, white and green colors.
This blue, as well as the ability for hydrangeas to change colors from red to purple to blue attracted attention from many scientists who were curious how such a thing could occur.
Research endeavors into the color-changing phenomenon of hydrangeas date all the way back to the late 19th- early 20th century in which it was found that mildly acidic soil levels appeared to contribute to the blue colors while more basic soil led to the color red. At least, this is what most people know about hydrangeas, and it has led hydrangeas to be titled “nature’s pH meter”.
However, it’s not just the soil acidity that is important, but also the aluminum content that is also pivotal in the formation of blue sepals. Aluminum generally exists in the soluble Al+3 ion in mildly acidic pH. At higher pH aluminum becomes insoluble in the form of aluminum hydroxide [Al(OH)3)] and with less available aluminum hydrangeas may appear red/pink.
It’s the availability of aluminum ions that appears to drive the blue color which occurs at these mildly acidic pH, usually around a pH of ~5 or below.
Information regarding these findings can be summarized within the Introduction of a paper from Ito, et al.1:
It is also well known that the color of H. macrophylla sepals change easily from red through purple to blue, depending on cultivation conditions. This phenomenon has been attracting the attention of not only plant scientists and horticulturist, but also flower lovers. The first reports about the strong correlation between soil acidity and blue color development1 and the role of aluminum ion (Al3+)2,3 date back to the early 19th century. In acidic soils (pH less than 5.0), the level of water-soluble Al3+ in soil is increased and absorbed by the roots. Once absorbed, the Al ions are transported into sepals where they form complexes with anthocyanin, resulting in blue sepal color. The structure of anthocyanins and the copigments in hydrangea sepals were reported in the mid-20th century4,5,6,7.
Again, the phenomenon of both blue sepals and color-changing sepals made hydrangeas appear rather unique, with an example of such a curiosity being mentioned in a paper from 19462:
The article appears to suggest that hydrangeas may not be entirely unique in their color-changing capacity, although it still makes for an impressive display, nonetheless.
Hydrangeas owe their colors to one unique anthocyanin in particular called 3-O-glucosyldelphinidin. This compound is part of a group of anthocyanins called delphinidins which are partially responsible for the purple and blue colors of flowers.
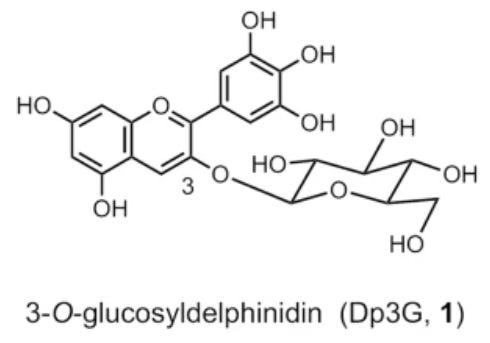
In general, only a handful of anthocyanins produce the color pigments found in flowers, with most of these color variations being derived from the number of hydroxyl groups added to the B ring of the base flavanol structure.
An example of this can be seen below when comparing delphinidin to two other anthocyanins- cyanidin (which elicits more purple) and pelargonidin (which appears orange). Again, the only difference between these anthocyanins is the number of hydroxyl groups as circled below.

It’s interesting to see how just a slight variation within the ring structure can elicit such drastically different colors. Just by modifying one compound ever so slightly you can cause a huge change in color display!
So, if such a process is simple why exactly are there very few blue-colored flowers in nature?
This is partially owed to the biosynthetic pathway for delphinidins which can be seen in the below diagram from Husain, et al.3:

Don’t focus on the details- the overall process is rather complex and is comprised of many different enzymes. In particular, different enzymes are needed in order to add on different hydroxyl groups onto the B-ring and produce the different anthocyanins seen, with one key enzyme being Flavonoid 3',5'-hydroxylase (F3'5'H). This enzyme is responsible for adding on the two hydroxyl groups onto the B-ring in order to form delphinidin.
What’s interesting is that many flowering plants, and especially popular ones such as roses, lilies, chrysanthemums, and carnations are deficient for the F3'5'H gene and therefore do not exhibit blue colors. It’s curious why such a gene would be lost for these plant species as a loss of blue should be balanced by some greater benefit.
Because many otherwise non-blue flowers make up the gardening market interest has led to research into genetically modifying these plants to elicit the blue color of flowers such as seen with blue chrysanthemums, which is usually done by way of inserting the F3'5'H gene into these plants or through alterations with other genes related to anthocyanin production in order to manipulate copigmentation coloration.4
However, it doesn’t appear that delphinidin exhibits a blue color by itself-several steps and factors are needed before we get to the actual blue color.
Instead, delphinidin can be converted into a quinodal base in which the middle hydroxyl is converted into a carbonyl group. This occurs in appropriate pH conditions and is a rather finicky structure, and therefore needs the help of other constituents in order to help stabilize the quinodal base.5 This effect appears to occur in several anthocyanins, however this phenomenon is showed explicitly for delphinidin below in which different pH and the respective structures are shown below (Yoshida, et al.)6

The ability to stabilize the quinodal base differs between flowers. For instance, some blue flowers such as dayflowers, which have been widely researched for their pigments, were found to form metal complexes between pigments and chelating ions in a manner similar to the one shown in the top right structure of the Yoshida, et al. figure but through the use of magnesium. This is where the aluminum in hydrangeas will come into play.
A rather interesting review on biosynthesis and genetic modification of flowers can be found in the review from Tanaka, Y., & Brugliera, F.7 from which I got some of my information for this article.
As it relates to hydrangeas there’s a bit more to the story as to how hydrangeas get their blue, which came from decades of research conducted by professor Kumi Yoshida and her team. A summary of their works can be found in their review from 2021, but what Yoshida and her team found was that aluminum appears to form a quasi-stable structure with 3-O-glucosyldelphinidin as well as other phenolic compounds such as 5-O-Caffeoylquinic acid acting as copigments, and it’s through this complex structure that hydrangeas get their blue:

This structure is referred to as being “quasi-stable” because this complex likely exists in equilibrium with its disassociated state (where the pigments or aluminum ions separate). The ability to form such a structure also depends on concentrations of delphinidin and other pigments, as well as aluminum concentrations and pH of the cell’s vacuoles. So, although the complex is stabilized by hydrophobic interactions it’s still rather labile and may explain why blue is rather elusive as it is a rather difficult color for many plants to maintain.
Overall, we can see that there’s quite a lot of work that is needed to elicit the color blue. Unlike other color pigments such as beta-carotene or quercetin there is no standalone pigment that can show as blue on its own.
Hydrangeas aren’t the only blue flowers out there, and if we are being technical, it’s not quite a “true blue” flower as it requires some manipulation through pigment/metal complex formation in order to obtain its hues.
Regardless, it doesn’t remove the fact that hydrangeas are beloved due to their vibrant blue colors as well as their ability to obtain a spectrum of colors all within the same plant.
It also serves to show that something as simple as a blue flower can be rather complex. There’s a lot to the research into blue flowers which I didn’t cover here- Yoshida’s work would be something of great interest for those who want to look even further. But even with the limited information examined we can see that it requires producing the right anthocyanin, having the anthocyanin exist in the right form, and having that form be stabilized by some sort of complex. It’s a lot of work to create a blue color, and it can make one appreciate how much work plants put into something that would otherwise seem simple.
Now, this article was originally going to look into some of the other colors of hydrangeas but unfortunately I came up empty on several fronts. For instance, you may be wondering about white hydrangeas- where do white hydrangeas come from?
I wasn’t able to find any clear information regarding white hydrangeas, but from the little information I came across white hydrangeas are seldom affected by soil pH and aluminum levels. They generally change color as the flowers age, sometimes faintly turning blue or pink, but should otherwise remain white.
Given the lack of dependency on soil pH and aluminum content it’s possible that white hydrangeas may have been bred to lack functioning enzymes needed for biosynthesis of these anthocyanins, and therefore it’s through a lack of anthocyanin pigmentation that the leaves appear white. This at least appears to be the case in some lily species in which transcription of genes related to anthocyanin pigmentation are reduced due to spontaneous mutations in some regulatory proteins.8
But there is also one question that has yet to have been answered- why is it that one hydrangea plant may display more than one color?

Unfortunately, it doesn’t seem that researchers have found an answer to that question yet. Maybe sometime in the near future Yoshida’s team will be able to find out what is going on.
Either way, enjoy the hydrangeas as they welcome us into the summer months!
If you enjoyed this post and other works please consider supporting me through a paid Substack subscription or through my Ko-fi. Any bit helps, and it encourages independent creators and journalists such as myself to provide work outside of the mainstream narrative.

Ito, T., Aoki, D., Fukushima, K., & Yoshida, K. (2019). Direct mapping of hydrangea blue-complex in sepal tissues of Hydrangea macrophylla. Scientific reports, 9(1), 5450. https://doi.org/10.1038/s41598-019-41968-7
CHENERY, E. Are Hydrangea Flowers Unique?. Nature 158, 240–241 (1946). https://doi.org/10.1038/158240a0
Husain, A., Chanana, H., Khan, S. A., Dhanalekshmi, U. M., Ali, M., Alghamdi, A. A., & Ahmad, A. (2022). Chemistry and Pharmacological Actions of Delphinidin, a Dietary Purple Pigment in Anthocyanidin and Anthocyanin Forms. Frontiers in nutrition, 9, 746881. https://doi.org/10.3389/fnut.2022.746881
Naonobu Noda et al. ,Generation of blue chrysanthemums by anthocyanin B-ring hydroxylation and glucosylation and its coloration mechanism.Sci. Adv.3,e1602785(2017).DOI:10.1126/sciadv.1602785
Funnily enough it appears that competing hypotheses arose during the early 20th century to explain blue colors in flowers. The initial hypotheses focused on pH alone as the compound cyanin was found in blue cornflowers as well as red roses. In order to assess the color variation across flowers researchers changed the pH of cyanin solutions and found that in acidic solutions cyanin gave off a red color while in alkali solutions it gave off a blue color. However, this argument was rebutted as a source of blue in plants since cells may not be able to maintain such drastic pH values, and eventually a metal complex hypothesis was made and substantiated when cyanin complexed to magnesium was found to exhibit a blue/green color, leading to research into examining pigment/metal complexes as a means of producing different colors.
Yoshida, K., Oyama, K. I., & Kondo, T. (2021). Insight into chemical mechanisms of sepal color development and variation in hydrangea. Proceedings of the Japan Academy. Series B, Physical and biological sciences, 97(2), 51–68. https://doi.org/10.2183/pjab.97.003
Tanaka, Y., & Brugliera, F. (2013). Flower colour and cytochromes P450. Philosophical transactions of the Royal Society of London. Series B, Biological sciences, 368(1612), 20120432. https://doi.org/10.1098/rstb.2012.0432
Suzuki, K., Tasaki, K. & Yamagishi, M. Two distinct spontaneous mutations involved in white flower development in Lilium speciosum. Mol Breeding 35, 193 (2015). https://doi.org/10.1007/s11032-015-0389-z



...the complexity of Blue. A great title for a book. Excellent read. Thanks!
Thanks! My wife Tina and I have several hydrangeas in our garden and they are a source of wonder for us. Ideally, perhaps, we would "dead-head" them as was done by the gardeners we no longer have. We leave them there and they age into a subtle decripitude, as we all do, if we last long enough.
I have never looked at the theory of dyes, but I guess that parts of the molecule resonate like a tuning fork. I guess that in this case, removing some hydrogens (not much of a loss of mass) and/or the bare oxygens somehow finding peace with a nearby captive aluminium ion makes the molecule resonate at a higher frequency, to accentuate blue wavelengths rather than lower frequency, longer, more green, yellow, orange or red waves.
This was a great antidote to the important but stressy corruptitude which is the main concern of most of the Substacks I read, not to mention https://zerohedge.com.