How rare are respiratory reinfections? Possibly not all that rare.
How recent unsubstantiated fear may not be rooted in evidence.
Edit 6/23/2022: The two Kaplan-Meier plots that were removed were added back.
Reinfections are one of those things I keep hearing about with increasing assertions.
It’s always about reinfections: reinfections, reinfections, reinfections!
And it’s something that’s been appearing with great frequency on both sides of the COVID discourse.
On one side, these vaccines are causing immune dysfunction and constant reinfection- something that supposedly shouldn’t happen.
On the other side pro-vaccine zealots are finding out that these vaccines aren’t the perfect gift that they’re made out to be, and now are finding ways to rationalize reinfection rates through the lens of more vaccinations (i.e. vaccinate to keep up with mutations).
The media appears to have taken up arms in screaming about reinfection as well.
Here’s one rather tame take on this reinfection drama, although I do find the need to post a “fact-checked” sticker to be somewhat unsettling.
There’s also this more recent article from the New York Times. And yes, I am using the Wayback Machine because why not (Actually I hit a paywall…)?
It’s interesting that, all around, people seem to have some vested interest in COVID reinfections and what may be causing it, including the mainstream press.
And it’s really the cause that serves the crux for much of this debate. But even as we speculate on the cause, we are all wondering if these reinfections are things that shouldn’t have happened, or whether this is just par for the course of SARS-COV2 but hasn’t yet been elucidated.
I’ll save some pontification for what’s going on saved for the end of the article, but first we’ll look to common viral infections and see if they provide any basis for the reinfection argument for SARS-COV2.
How common are cold and flu reinfections?
In order to answer the question of if reinfection with SARS-COV2 is expected, we should turn to seasonal viruses and see what the literature offers on these topics.
Before COVID, I never questioned how long immunity against the flu lasted, even if I got the annual flu vaccine. I generally knew that I may get the flu every season, and that should at least provide some anecdote towards prior immunity knowledge. That is to say, we probably should have known better than to make such bold assumptions about long lasting immunity against a respiratory infection when many of us know full-well that we can get either the flu or cold every year.
Anyways, enough of that early pontification and a look at a few studies.
Okay, a bit of a slight tangent: what’s strange is that the literature seemed to have made this false assumption about seasonal respiratory infections as well- that they should lead to long, durable immunity. So unfortunately there doesn’t appear much in the ways of robust studies, but we’ll make do with what little is available.
One study comes from a 1983 UK1 longitudinal study which looked at H1N1 influenza reinfection among UK boarding school boys. In 1978 H1N1 first emerged after decades of absence and led to a large infection of most of the boys housed:
There was an outbreak of influenza caused by influenza A HlN1 in February 1978 when there were 420 clinical cases. It was estimated that 90% of all the boys in school were infected (Davies et al. 1982). In the Spring of 1979 there was a further small outbreak caused by this subtype, which affected mainly those boys who had joined the school in the previous autumn. However, there were a few reinfections (some with associated symptoms) in those who had been present during the earlier outbreak. In February 1983 another outbreak occurred with approximately 200 cases caused by influenza A H1N1.
The researchers of this study conducted routine sera collection from 29 of the boys who were boarded at the school (these boys were there for multiple years) in order to check for seropositivity (antibodies against the flu) and against which influenza strain. Out of this subset of students initial evidence suggested that 27 of them were infected by the 1978 H1N1 outbreak (the other 2 were not part of the boarding school at the time) and successive years saw minor reinfections of some of these boys, including another outbreak of H1N1 occurring in 1983. Interestingly enough, none of the boys were spared from reinfection after the 1978 H1N1 outbreak (emphasis mine):
Between 1979 and 1983 there were a further four reinfections. The fate of all these boys in 1983 is shown in Table 1. Cases were assessed by examination of paired sera collected at onset of symptoms and approximately 2 months later. Infection in boys without symptoms was determined by examining sera collected in April 1982 and April 1983. The case and infection rates in the two outbreaks are compared in Table 2. The infection rate was high on both occasions but there was a marked difference in the clinical to asymptomatic infection ratios. During the 5 years 1978-83 not one of the 29 boys escaped infection. Two boys had been infected once, 24 boys had been infected twice and there were three boys who had been infected three times.
Even though there were reinfections with the 1982 H1N1 outbreak, there were fewer incidences of symptomatic infection as compared to the 1978 infection, suggesting that homology between the two outbreak strains may have provided some form of protection. The researchers compared this information to similar outbreaks of H3N2 in Hong Kong that suggested similar results. Interestingly it appears that the boys were not protected from exposure to other strains of influenza, with a remark that antigenic drift is likely to play a role in reinfection protection (before we start trying to analyze this for any OAS, I will argue that this study is severely limited and people should be careful from extrapolating too much from this observational study).
However, the researchers do provide a little comment about antibodies that is rather interesting (Note that there may be a few typos in dates below. Or maybe I’m mixing up the dates. Regardless, I’m not quite sure what exact date the antibodies were no longer detectable.):
Only six of 26 boys infected with H1N1 in 1978 produced antibody which reacted with the 1983 outbreak strain and this was no longer detectable by 1982.
Aside from the weird dates, we can see that the antibodies may not be long-lasting, but the overall immunity may last for an extended period. Maybe there’s more to immunity than just antibodies? Hopefully that comment doesn’t come off as too heretical!
This study was interesting, but considering this occurred over the course of a few years, and that the second outbreak occurred 4 years after the first one doesn’t provide much commentary on the possibility of 2 or 3 infections with COVID a year. Granted, the researchers do note evidence of reinfection in the years between the two outbreaks. Nonetheless, this study does suggest that a few reinfections are possible (barring any examination of heterogeneity among the boys).
One study that better provides such a perspective is an observational study from Galanti, M. & Shaman, J.2
Just like many of us, these researchers wondered whether SARS-COV2 reinfection was possible, and thus turned to seasonal coronaviruses for possible evidence:
As the pandemic progresses, infecting millions of people across the world, a key question is whether individuals upon recovery are prone to repeat infection. There have been reports of individuals again testing positive by polymerase chain reaction (PCR) weeks after recovering from a SARS-CoV-2 infection. However, in Korea, as reported by the Korean Centers for Disease Control and Prevention, viable SARS-CoV-2 was not isolated in cell culture of respiratory samples from potentially reinfected individuals [5]; thus, these subsequent positive results may have been due to inactive genetic material detected by molecular testing. A recent animal challenge study showed evidence of (at least) short-term protection against reinfections in rhesus macaques experimentally reinfected 4 weeks after first infection [6]. […]
Reinfections with the respiratory viruses have been reported in previous studies, in which individuals were infected in 2 sequential challenges with the same H1N1 virus [7, 9]. Studies focusing on respiratory syncytial virus have provided evidence of subsequent reinfection with very similar strains or with the same strain in <1 year [10, 11]. Serological studies have documented subsequent infections with endemic coronaviruses [12]. Sequential rhinovirus infections have also been reported in a number of studies; however, this finding could be due to the multitude (>150) of antigenically distinct types of rhinovirus in circulation [13].
The researchers took sampling data from 214 individuals who participated in a Virome project. The project was intended to conduct proactive coronavirus data collection among those living in various boroughs of Manhattan. The study occurred between October 2016 and April 2018.
Researchers collected mandatory weekly nasopharyngeal swabs irrespective of the participant’s symptoms in order to conduct PCR tests for infections. Participants also filled out daily self-reports answering questions about their symptoms.
Among all of the participants enrolled in this project 86 tested positive at least once within the timeframe provided. The researchers broke down which strains of coronaviruses participants were infected with, but the most important part of the study for our discussion is the one that examined reinfections.
The authors comment on the reinfection rates of some of these participants:
Among the study participants, 12 individuals tested positive multiple times during the study for the same coronavirus: 9 tested positive multiple times for OC43, 2 tested positive twice for HKU1, 1 tested positive twice for 229E, and none tested positive multiple times for NL63. Among the 9 participants with multiple OC43 infections, 3 individuals experienced 3 separate infection episodes, and the other 6 experienced 2 separate episodes. The median time between reinfection events was 37 weeks. The shortest time for a reoccurrence of infection was 4 weeks (OC43), and the longest was 48 weeks (OC43). Among the 12 individuals testing positive multiple times for the same coronavirus, 9 were children aged between 1 and 9 years at enrollment, and 3 were adults aged between 25 and 34 years (see Supplementary Table 4 and Supplementary Figure 1 for characteristics and timelines of the repeated infections).
And provide a Kaplan-Meier plot3 which estimates the probability of reinfection with the same betacoronavirus after an initial infection.
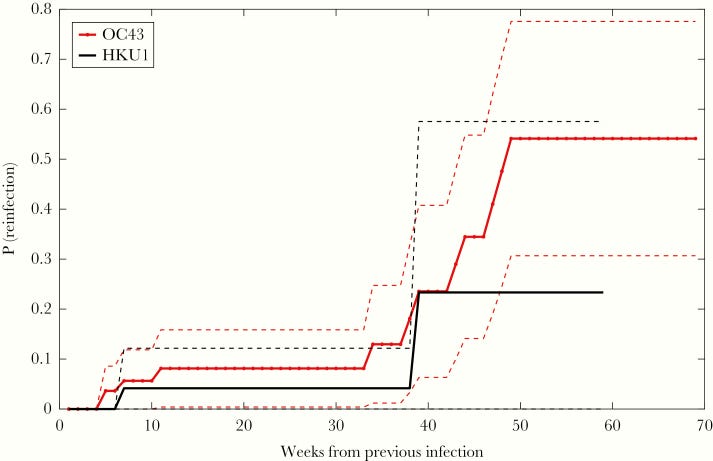
Note that this graph uses very limited data (i.e. only 2 reinfections from HKU1 occurred during the time of this study) and shouldn’t be looked at too extensively for other implications. Instead, view this graph as an indication that reinfection from a coronavirus infection is possible within a year of the first infection.
The researchers provide this comment in their excerpt in regards to their results, with a possible explanation about their findings:
Nevertheless, this study confirms that reinfections with the same coronavirus type occur in a time window shorter than 1 year, and finds no significant association between repeat infections and symptom severity. Instead, it suggests the effect of possible genetic determinants of innate immune response, as individuals asymptomatic during first infection did not experience symptoms during subsequent infections, and members of the same families reported similar symptom severity. Genetic variations associated with immune responses have been associated with increased severity and exacerbation of symptoms due to respiratory infections [30, 31].
So the authors suggest the importance in considering independent differences (genetic diversity) among individuals and how this could alter ones chances of reinfection. The authors of this study didn’t examine this factor, but it’s also something that should be taken into account- we all produce different immune responses, and understanding why we do should be critical when considering reinfection.
Overall, the study from Galanti, M., & Shaman, J. was relatively small and cast doubt on the significance of this study in the broader context, and certainly with respect to SARS-COV2.
In one much larger study from Petrie, et. al.4 researchers took to analyzing reinfection data from a large household study titled the Household Influenza Vaccine Evaluation (HIVE) study, which was a study that examined influenza infections within Michigan households with vaccinated family members and children in order to evaluate vaccine effectiveness. The study is a joint partnership between the University of Michigan School of Public Health with funding from the National Institutes of Allergies and Infectious Disease (NIAID).
The data collected was derived from the years 2010-2018 and also included data on infections such as human coronaviruses, so the researchers used this information as a reference to the possibility of SARS-COV2 reinfections:
Over that period, 2010–2018, 1004 infections were detected by reverse-transcription polymerase chain reaction (RT-PCR). The infections were most frequent in children, but substantial numbers of infections were identified in adults. This, and past studies of HCoVs [human coronaviruses] suggested that these agents, like most respiratory viruses, reinfect through life [12, 13]. In this report, we characterize RT-PCR–documented, symptomatic reinfections with these viruses and investigate antibody response to infection, including cross-reactivity and persistence.
We’ll avoid the discussion about antibodies and cross-reactivity and focus solely on the reinfection evidence.
As mentioned above, out of the nearly 3,418 participants in the HIVE study over 1004 human coronavirus infections were detected within the given timeframe. Out of these 1004 infections, the researchers noted a high level of reinfection among the participants (note the unfortunate person who apparently had 13 reinfections!):
HCoV reinfections were common in the cohort (Figure 3; Supplementary Figure 1). Overall, the 1004 ARI [acute respiratory infections] with HCoV occurred among 701 individuals; 303 (30%) of these represented documented reinfection. The number of ARI episodes with HCoV infection ranged from 1 to 13 per individual (Figure 1). Of the overall 303, 81 any type reinfections were identified in the same study year (1 July–30 June), a relatively short period given their seasonality. Of the 81 reinfections during the same study year, 12 were of the same HCoV type potentially representing prolonged shedding (range of days between illnesses, 14–152). Considering any type reinfections, the mean time to reinfection was 505 days. The mean time to same type reinfection was estimated at 983 days for 229E, 578 days for HKU1, 615 days for OC43, and 711 days for NL63 (Supplementary Table 3).
The researchers conducted their own Kaplan-Meier plot in which they measured the number of participants who were not reinfected. Of course, as time went on fewer and fewer participants were not reinfected by a coronavirus infection (apologies for the super tiny font on the graph!).
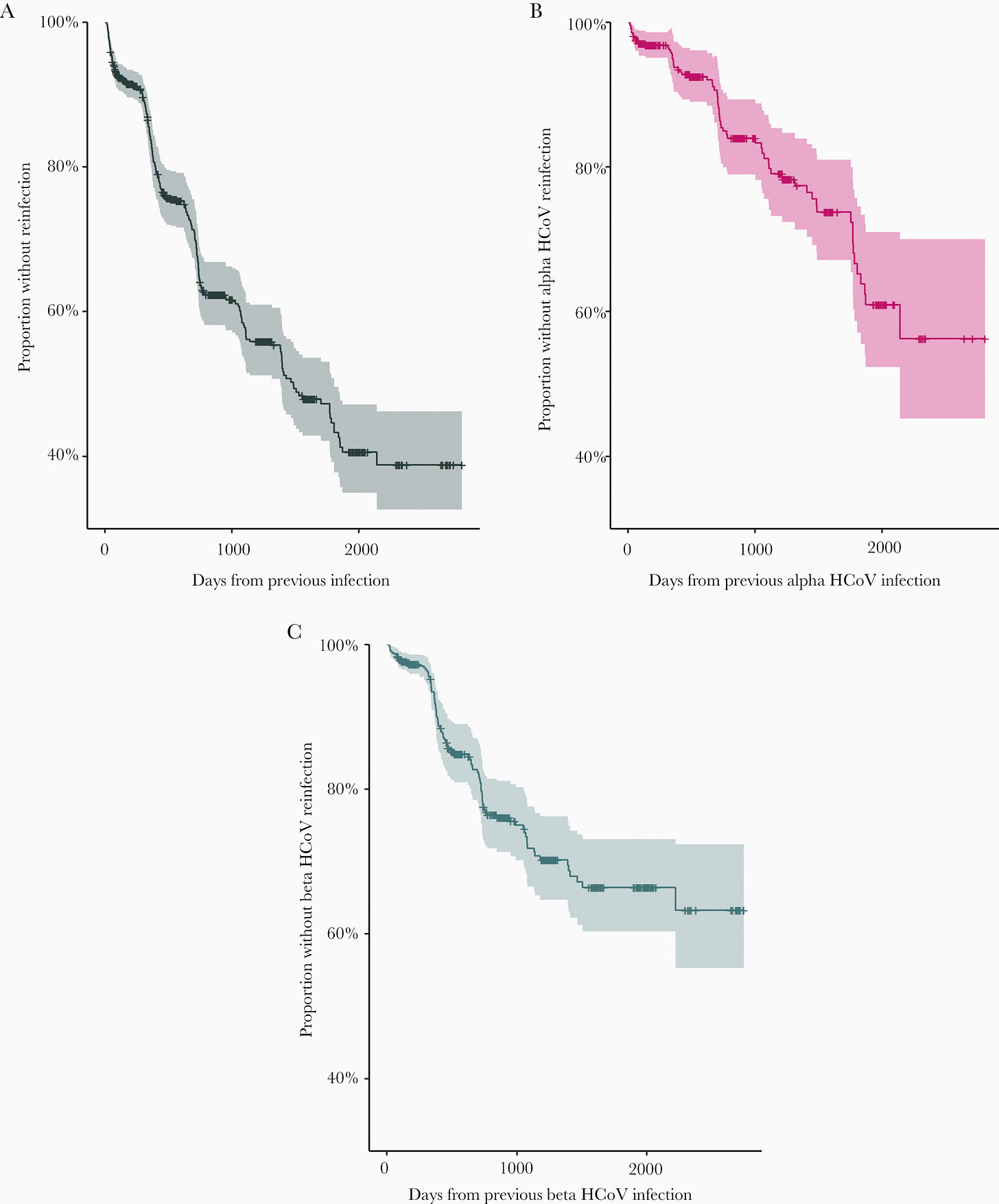
The mean time of reinfection was around 505 days, or more than a year and a half after the first infection, which may not provide a reasonable time reference for SARS-COV2. Keep that in mind with many of these studies, and also keep in mind that many of these studies are observational and are likely to miss multiple confounding variables.
The authors make this conclusion from the reinfection portion of the study (emphasis mine):
While it was clear from studies conducted years ago that reinfection with seasonal HCoVs, like other common respiratory viruses, occurred throughout life, their frequency had not been a matter of great interest [12, 13]. This has become more urgent currently given the importance of knowing how long immunity might last after SARS-CoV-2 infection and vaccination. Given the age structure of our cohort, and high levels of preexisting antibody, nearly all infections observed in this study are likely reinfections. However, nearly a third of all identified infections were confirmed reinfections during enrollment with an average duration of 505 days between infections. In primary analyses, we observed that the risk of infection with beta genus coronaviruses was higher if an individual had a beta coronavirus infection in the immediately prior study year. This effect was attenuated in sensitivity analyses conditioning only on those with a prior infection, suggesting that this result is likely due to confounding by unmeasured shared risk factors for infection rather than a specific biological effect. Regardless, this finding underscores the frequency of reinfection in this cohort. […]
This study has demonstrated that seasonal HCoV reinfection frequently occurs over a relatively short time period and is possibly affected by the prior infecting virus type. However, we have not determined precisely how frequently this occurs in a broad population nor is it evident whether this will apply to SARS-CoV-2 reinfection or infection after vaccination. Still, it does suggest that duration of immunity will probably be limited by either waning immunity or viral antigenic drift, at least in some of the population, and supports careful determination of the need for booster vaccinations as time from original immunization increases.
So far we have seen observational evidence that suggests reinfection with respiratory viruses is not actually a rare phenomenon. There are many issues with these observational studies since they indicate reinfection many months after the initial infection. They also relied on PCR tests with patients providing information on their own symptoms to confirm infection, so there are issues as to how accurate some of these studies are (collection of antibodies should at least provide further validation of infection).
What we may need for direct evidence of reinfection is a study in which participants were intentionally infected.
Such a study is considered a challenge study since the subjects are provided a virus. You may have noticed the term challenge be used extensively in many animal studies where mice or rats were given a virus.
Instead of rats, there is one study which looked at a very small sample of participants and challenged them twice with the influenza virus to examine flu reinfections.
This study from Memoli, et. al.5 was two separate studies in which participants were challenged with the influenza A virus. The studies were conducted at different time points, and the researchers noticed that 7 of these participants enrolled in both challenge studies. Thus, they used these 7 people as a measure of reinfection:
As part of a dose-finding and validation study, 46 volunteers with variably high and low preexisting HAI titers against the A(H1N1)pdm09 virus were challenged intranasally [10]. In a subsequent challenge study using the identical challenge virus, 7 of those original 46 volunteers were rechallenged approximately 1 year later [11]. In this substudy, we evaluate and describe the outcomes of these 7 rechallenge participants and discuss what these results may suggest about correlates of protection and development of more broadly protective influenza vaccines.
These two challenge studies took relative healthy participants and challenged them with an influenza A viral load intranasally. Then, 7 of those participants were challenged again with an average rechallenge time under 1 year (337 days; range of 7.5-18.5 months).
The results of the 1st challenge are found below:
In challenge 1, all 7 participants developed signs/symptoms consistent with experimental influenza infection. Five of the 7 demonstrated objective laboratory evidence of experimental influenza infection: 3 of the 5 had detectable viral shedding, all 5 demonstrated HAI seroconversion, and 4 of the 5 demonstrated NAI seroconversion. […] Two challenge subjects with very mild or moderate symptom scores (participants 2 and 7, Table 1), had neither viral detection nor seroconversion. Of note, the 3 challenge 1 participants with both viral shedding and seroconversion also showed the strongest increases in PBL antiviral, T-cell, and B-cell gene expression acutely (Figure 1), and developed the most significant influenza-related symptoms over a 5- to 11-day period (Table 1), with tabulated symptom scores above the mean for challenge 1 [10, 11].
The results of the second challenge are found below:
During challenge 2, 5 of 7 patients developed signs/symptoms consistent with influenza infection (Table 1), and 6 developed objective laboratory evidence of infection. Four of the 7 participants (1, 2, 3, and 7) demonstrated detectable viral shedding of the identical challenge virus to which they had been previously inoculated in challenge 1. […] While 5 of the 7 challenge 2 participants exhibited influenza-related symptoms (participants 3–7), the remaining 2 participants, who both had detectable viral shedding, were asymptomatic (participants 1 and 2). Of the 5 participants who had objective laboratory evidence of influenza infection in challenge 1, 4 demonstrated similar objective evidence of infection in challenge 2, as documented by viral shedding and/or seroconversion. One additional subject (participant 4), who had clear laboratory evidence of infection in challenge 1, experienced 4 days of mild influenza-like illness without detectable viral shedding or seroconversion.
This study was very interesting because it was an actual human study that challenged participants with both an initial influenza infection as well as a reinfection. What’s more interesting is that many of these participants who were rechallenged also presented with flu-like symptoms.
Overall, this study- albeit very small- raised prior questions as to whether respiratory infections actually led to robust, long-lived immunity as was originally thought:
The 7 influenza subjects evaluated here represent a group of healthy individuals who were exposed to the identical influenza A(H1N1)pdm09 virus in 2 consecutive challenges. This is a unique circumstance: In nature, most individuals are probably relatively protected from reinfection with the same or different influenza strains and subtypes for several months after infection [15] and may not typically be reexposed to identical or closely related influenza viruses until a subsequent influenza season, at which time circulating viruses may be antigenically drifted.
While it has long been known that vaccine-induced protection wanes after 6–12 months [9, 16], it has seemed reasonable to many observers that homotypic protection against a natural influenza virus infection should be long-lasting. However, the observations reported here, that persons can be infected with a specific influenza A virus, mount an immune response against that virus with or without influenza signs and symptoms, and then be reinfected with the genetically identical virus after about 1 year’s time, raises questions about the existence, nature, and kinetics of long-term protective immunity and correlates of protection.
The clearest example of reinfection may be participant 3, who developed nearly equivalent clinical disease and detectable viral shedding after both challenges (Table 1). One other subject (participant 1) also demonstrated viral evidence of sequential infection, but with much less clinical illness during the second infection. The other 5 individuals (participants 2, 4, 5, 6, and 7) also demonstrated clinical signs and symptoms supportive of sequential infection (Table 1).
So here we don’t have just evidence of observational reinfection, but of an actual clinical study indicating that reinfection is possible with respiratory infections. These studies may not tie directly with SARS-COV2, but it at least suggests that our prior understanding of immunity may be heavily flawed, or at least conflated.
But we should have at least known about this before, mostly because we know anecdotally that seasonal infections of the cold and flu are quite common, so why would we think otherwise with SARS-COV2?
Has there been a messaging problem?
As talks of vaccines emerged the initial discourse was a battle between natural immunity and vaccine-induced immunity. Many arguments suggested that natural immunity was the best, and that it shouldn’t lead to additional infections. Dr. McCollough made this claim on Joe Rogan, but revised his argument to suggest that Omicron has upended this thought (Dr. Malone corrected this issue when he went on Joe Rogan’s podcast afterwards). And so the initial argument indicated that natural immunity should have led to some form of sterilizing immunity.
But this appears to not be the case, and in reality there actually existed some evidence that respiratory infections don’t provide long-term immunity.
Take a look at this review from Yewdell, J. W.6 who argues that herd immunity against SARS-COV2 would be very difficult:
If immunity to SARS-CoV-2 and seasonal CoVs are similar, COVID-19 herd immunity is a pipe dream, even more so given the relatively rapid selection of mutants with amino acid substitutions in the spike protein that reduce the efficiency of serum antibody neutralization [6]. Absent effective herd immunity, over the next few years, individuals can choose whether their first exposure to SARS-CoV-2 immunogens occurs via vaccination or infection. With the age-related increase in COVID 19 severity, it is critical that individuals be vaccinated sooner rather than later.
Coronaviruses are hardly unique in their ability to reinfect humans. Infection with none of the common endemic human respiratory viruses consistently induces durable immunity (Table 1). Although influenza A and B viruses are notorious in this regard, they are, in a sense, less adept than the other respiratory viruses, which reinfect individuals without resorting to significant antigenic variation. Similarly, many viruses that infect the gastrointestinal (GI) tract can infect vaccinated or previously infected individuals, most likely due to waning immunity (Table 1). Unlike respiratory viruses, however, several GI viruses are well controlled by infection or vaccination, including poliovirus, now on the brink of vaccine-induced extinction.
Now, aside from the vaccine comment, the argument above suggests that SARS-COV2- if it behaves anything similar to common colds and seasonal flus- could possibly lead to reinfections (this was the argument raised in the NYT and Bestlife article above).
The table provided by Yewdell, J. W. looks specifically at viremic pathogens (pathogens that may travel in the blood), but it highlights how most respiratory infections don’t lead to long-lasting immunity.
Now, there is some evidence that suggests natural immunity is longer lasting than vaccinated immunity: a recent study from Qatar may point to this happening with SARS-COV2. But remember that this is relative, such that relative to vaccinations naturally immunity may last longer.
The only question is how long is longer, and how long is good enough to prevent reinfection?
Prior evidence suggests that infection with the 2003 SARS-COV infection may not have led to long-term immunity7, and so even a very similar relative to SARS-COV2 doesn’t substantiate these claims.
So how did this argument of durable natural immunity arise? Were we possibly misled in assuming that sterilizing immunity was the default setting with SARS-COV2?
I don’t really have any answers for these, although part of me suspects that the overuse of the term “novelty” may have contributed in some way to our current predicament by removing any prior knowledge of viruses and immunology due to “novelty”.
I also find it strange how we’ve begun to rely on PCR and Antigen tests as measures of infections when months ago we tended to argue that these tests were either widely inaccurate are far too sensitive. As someone who has done COVID PCR testing I have previously remarked that the tests are generally too sensitive for widespread use. Now, some people may present with a positive test and symptoms, but when they’re mild it just begs the question as to what constitutes mild, and whether we are testing for viral particles while symptoms may come from other sources.
It also doesn’t help that the literature was lacking in this information as well. Several of the studies listed above only came about during the COVID pandemic, and they only did so as a way of elucidating reinfection plausibility with SARS-COV2.
So essentially, a lot of the confusion and hysteria coming about now may just be a consequence of lack of knowledge or improper use of terms. We may have considered lasting immunity to be some abstract truth since we are trained to assume that to be the ideal case. Instead of examining the veracity of these hypotheses when they falter, we may instead argue that these hypotheses are the truth, and that deviations from this truth may actually point to something nefarious at play.
All in all, I'm not quite sure why such a widespread override of prior knowledge has led many of us to become surprised with the recent predicament. Keep in mind that many factors have been left out of this discussion such as the role of mucosal immunity, severity of illness, and the difference between Omicron and prior variants.
What I can say is that the evidence so far suggests (doesn’t prove) that SARS-COV2, like other respiratory infections, may (big MAY- we don’t have any evidence outside of anecdotes and observations) lead to reinfection down the line. What this means for the future? I don’t know, nor will I pontificate about any nefariousness or the possibility of COVAIDS- I think there’s far too much immunology word salad going on already.
But hopefully this post sheds some light into what we are dealing with right now, and provides some much needed context during this bout of uncertainty and hysteria.
If you enjoyed this post and other works please consider supporting me through a paid Substack subscription or through my Ko-fi. Any bit helps, and it encourages independent creators and journalists outside the mainstream.

Davies, J. R., Grilli, E. A., & Smith, A. J. (1984). Influenza A: infection and reinfection. The Journal of hygiene, 92(1), 125–127. https://doi.org/10.1017/s002217240006410x
Galanti, M., & Shaman, J. (2021). Direct Observation of Repeated Infections With Endemic Coronaviruses. The Journal of infectious diseases, 223(3), 409–415. https://doi.org/10.1093/infdis/jiaa392
Kaplan-Meier plots are used extensively in drug trials to measure outcomes. These are typical of studies that may mention something such as “28 days post intervention” such as in the Remdesivir trials. For more information here’s another paper on Kaplan-Meier plots:
Rich, J. T., Neely, J. G., Paniello, R. C., Voelker, C. C., Nussenbaum, B., & Wang, E. W. (2010). A practical guide to understanding Kaplan-Meier curves. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery, 143(3), 331–336. https://doi.org/10.1016/j.otohns.2010.05.007
Joshua G Petrie, Latifa A Bazzi, Adrian B McDermott, Dean Follmann, Dominic Esposito, Christian Hatcher, Allyson Mateja, Sandeep R Narpala, Sarah E O’Connell, Emily T Martin, Arnold S Monto, Coronavirus Occurrence in the Household Influenza Vaccine Evaluation (HIVE) Cohort of Michigan Households: Reinfection Frequency and Serologic Responses to Seasonal and Severe Acute Respiratory Syndrome Coronaviruses, The Journal of Infectious Diseases, Volume 224, Issue 1, 1 July 2021, Pages 49–59, https://doi.org/10.1093/infdis/jiab161
Memoli, M. J., Han, A., Walters, K. A., Czajkowski, L., Reed, S., Athota, R., Angela Rosas, L., Cervantes-Medina, A., Park, J. K., Morens, D. M., Kash, J. C., & Taubenberger, J. K. (2020). Influenza A Reinfection in Sequential Human Challenge: Implications for Protective Immunity and "Universal" Vaccine Development. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 70(5), 748–753. https://doi.org/10.1093/cid/ciz281
Yewdell J. W. (2021). Individuals cannot rely on COVID-19 herd immunity: Durable immunity to viral disease is limited to viruses with obligate viremic spread. PLoS pathogens, 17(4), e1009509. https://doi.org/10.1371/journal.ppat.1009509
Tang, F., Quan, Y., Xin, Z. T., Wrammert, J., Ma, M. J., Lv, H., Wang, T. B., Yang, H., Richardus, J. H., Liu, W., & Cao, W. C. (2011). Lack of peripheral memory B cell responses in recovered patients with severe acute respiratory syndrome: a six-year follow-up study. Journal of immunology (Baltimore, Md. : 1950), 186(12), 7264–7268. https://doi.org/10.4049/jimmunol.0903490







Thanks for a good review. Unsubstantiated fear is never rooted in evidence. But media makes its living by promoting fear (as do politicians), so the headlines will continue to do so, regardless of the lack of science. Media is in the business of selling advertising after all. Not in presenting evidence.
Just one limited comment: Is there anyone left who does NOT realize the fear in NOT "rooted in evidence" ? Fool me once, shame on me, fool me twice.......