Everyone is Getting COVID. It’s time to learn and deal with the virus.
PART I: Understanding PCR and Rapid Antigen Tests
I thought I would not write about Omicron for a while, especially after writing one of my longest series and uploading it just recently.
Well, as I pointed out to you all on Friday, and possibly due to some form of cosmic justice that I would rather not question, it finally happened; I caught Omicron.
Surprisingly (or not), my symptoms were mild. I had mild sore throat, and a headache that may be more related to all the tea I was constantly drinking rather than the illness. I did in fact lose my taste and smell which is just beginning to come back so I would attest to having indeed had COVID.
Omicron has truly changed the landscape of COVID. It’s not behaving like any other strain of the disease. Fortunately for us, it means that it is presenting with more mild illness; a great benefit for us as a species considering how quickly it is spreading.
But even though many people are experiencing mild symptoms with Omicron, other people may not be so fortunate to have such mild symptoms, even if they have been both vaccinated and boosted. Even still, many people may still prefer a more speedy recovery and want to find options to help reduce their symptoms.
Regardless, rational thinking seem to still be missing among many of our policies and approaches to COVID.
Because everyone is getting COVID, it seems appropriate to provide a few bits of information to help inform people about the tests that are being deployed, what they can possibly do at home, and why there still continues to be several gaps within our COVID logic.
The first section will provide some information about both Antigen and PCR tests.
PCR vs Antigen Tests
There are two forms of COVID tests available; an Antigen test and a PCR test. Both operate by detecting the presence of SARS-COV2, although they do so in distinct manners, which we will cover in brief detail. But before that, we’ll go over briefly two important concepts that these tests, and really many other testing procedures operate on; accuracy and precision.
Accuracy and Precision in Testing
There’s been a lot of heat around both Antigen tests and PCR tests. You may have heard that people refer to Antigen tests as being “less accurate” than a PCR test. You may have also heard PCR tests referred to as being “far too sensitive”- I have made those sentiments known on my Substack. Here, I’ll provide some context and information about what these phrases refer to in regards to tests.
Accuracy and precision both refer to ways of examining error when conducting scientific studies or experiments, although it is likely to be used in many other areas as well. Accuracy refers to how close the measurement or result you see is what you should expect. Essentially, if you see a positive COVID test result how likely is the result you see the actual result? Precision refers to consistency, such that after continuous tests how likely are you to get the same result across all of the tests?
If you’ve ever taken a statistics class, or any introductory science course you’re likely to have come across the “dartboard” metaphor used to illustrate accuracy and precision.
Now, let’s take a typical dartboard. In such a game as darts, the intent to is to hit as close to the center target on a dartboard as possible. We also assume that every dart thrown at the dartboard is done so with the intent of hitting the center dot. Thus, how close someone gets to the center is referred to as how “accurate” of a throw someone had. For our sake, when speaking in terms of COVID tests, the concept of accuracy refers to how likely the result displayed by a test is indeed the correct result.
Let’s say that you throw a few more darts at the board. Whether or not you hit the center, you notice that they tend to collect in one area of the board (such as the left side). Because all of the darts you threw skewed in a certain direction, these throws would be considered consistent i.e. precise.
In science, measurements need to have great level of both accuracy and precision. Not only does it tell you that you can trust the results you get, but that you should continuously expect to get similar results.
Being both accurate and precise are extremely important, but sometimes limitations in testing procedures or the type of assay does not allow for a test to either be fully accurate or precise. This presents with 4 possible scenarios, which are shown in the dartboards below.
Precise but not Accurate (Scenario 1): Let’s say you decide to use your oven to bake cookies. You set the oven at 350 Fahrenheit, and the recipe says to bake them for 12 minutes. After 12 minutes, you notice your cookies are underdone and require a few more minutes. However, you notice that your oven constantly runs colder than the temperature you set whenever you use it. Calling an electrician, you find out that your oven constantly runs 15 degrees below the display temperature. Here, this scenario would be considered inaccurate (the actual temperature is not the same as the display temperature) but it would be considered precise (the oven is consistently 15 degrees below the set temperature). In this case, this is a slightly different form of precision, but it still outlines the topic of consistency, and more importantly, predictability in regards to the temperature. In testing procedures, this type of precision is important as it indicates that errors will tend to skew a certain direction, such that a test will consistently either test as a false positive or a false negative if the test is not highly accurate. This provides a level of predictability that can be used to interpret a test’s result.
Accurate but not Precise (Scenario 2): It’s a new year, and so many people are probably thinking about changing a few lifestyle habits such as dieting and exercising. In this scenario, someone decides to buy a new scale. They find that their old scale constantly measured around 180 lbs. (not my personal weight...), so you have a baseline to work with for the new scale. This person decides to weigh themselves, and gets 180 lbs. However, this person decides to weigh themselves a few more times and gets the following results: 175 lbs., 185 lbs., 187 lbs., and 173 lbs. In this case, the results are not consistent, so these results would not be considered precise. However, they all average around the actual value of 180 lbs., and thus would be considered accurate. This is a difficult scenario, especially when it comes to science measurements or test accuracy and precision. If a test is not precise but accurate, you are likely to get a correct result out of several attempts, although it would be more difficult to figure out which one would be the correct result to accept. More importantly, the lack of consistency means that there is no predictability to how the data will skew. Take the scale example above, you may sometimes be under or over the expected weight. There really is no way to determine your actual weight with an imprecise scale. In such a scenario, several tests may be conducted to average out to an accurate test, although the practicality of such a test should rightfully be brought into question. There’s no feasible way to provide someone with 5 tests where the results are averaged to determine the positivity of a patient.
Neither Precise nor Accurate (Scenario 3): This is all-around a bad scenario. Not only would you not be able to tell if your results are what they should be, but you can’t even determine if you will get consistent results! This is an entirely lose-lose situation. If a COVID test was designed with such errors, there would be no way that such a test would gain either FDA or EUA approval. Essentially, your assay would be considered no good! Be wary of this notion when it comes to tests. Although both Antigen and PCR tests are likely to have incidences of both false positive and false negative tests, these tests are far more likely to skew more towards one error than another. Again, a test that receives both errors at high rates is not a test that would be considered good by any measure!
Results are both Precise and Accurate (Scenario 4): This is the best scenario, and like I previously mentioned it indicates that a test provides a reliable result that is both highly likely to be the actual result as well as a consistent result.
Overall, the best test would be one that carries both a high degree of accuracy as well as a high degree of precision. Under no circumstances would a test that provides neither be considered a viable test. That leaves us with the other two options, and the choice of whether some level of accuracy should be sacrificed for precision comes down to what type of assay is being conducted, although it should be noted that the idea of sacrificing some level of accuracy for precision is likely to be the best compromise.
Before we dive into each individual test I would just add an additional note on what’s called test sensitivity. A test’s ability to provide a result depends entirely on the level of whatever substance is being measured. In the case of COVID tests, these are SARS-COV2 viral levels. Whether Antigen or PCR, each test requires a certain level of virus before the assay is able to pick up that any viral particles are there. This is called the limit of detection. Both the limit of detection and test sensitivity are inverses of one another. A test that is highly sensitive requires very little viral particles to give a result while a test with lower sensitivity will require a higher level of viral particles, and thus a higher limit of detection. Keep these two concepts in mind, as they play large roles into the differences between each test.
PCR Tests- A Brief Overview
I’ll start off with PCR tests. They’re the ones I have the most familiar with having previously done COVID testing. It’s also the type of test that I’ve written about several times on my Substack, so I will keep it concise here and refer people to my prior posts.
In short, PCR stands for Polymerase Chain Reaction, and it refers to the amplification technique used to acquire detectable levels of amplified DNA called amplicon. Usually the genetic material must first be extracted from its source. For SARS-COV2, nasal swabs are collected and stored in a solution, although some testing facilities may utilize oral/buccal swabs, or even saliva without requiring swabs. The virus’ RNA is first extracted and isolated. Then, during the PCR portion of the run the isolated RNA is combined with several reagents including primers, which are fragments of DNA that attach to regions of the viral genome that provide a starting point for the polymerase to amplify the specified gene. In the case of RNA, the RNA must first be converted into DNA before the amplification occurs because the constant cycling is likely to degrade RNA at a much faster rate than DNA.
When PCR is utilized as a diagnostic tool for viral infection, the specific technique used is referred to as real-time PCR (rt-PCR). Unlike a typical PCR assay which is more of a “set it and forget it” form of amplification, rt-PCR allows technologists to examine the amplification process as it occurs, and it provides results at a critical PCR point rather than waiting until the end where the data may be more heavily influenced by testing biases.
Because every cycle of PCR amplifies the DNA the amount of amplicon produced follows an exponential curve, such that each cycle leads to an exponential increase in amplicon by nearly doubling the amount of material after each cycle. Also, because a very small amount of virus is needed due to the amplification process, PCR tests are considered highly sensitive.
PCR tests have become the staple for COVID testing as they are considered to be highly accurate. However, their high level of sensitivity has brought into question whether the results of these tests are not being analyzed properly.
Although it’s not usually discussed, the Ct Cutoff point for the rt-PCR process is based off of initial tests of infectious viral load taken at the beginning of the pandemic. The number of cycles needed to reach that cutoff can essentially be referenced to find an initial viral load of the patient, which can then be interpreted to be an infectious load due to the supposed amount of viral particles. As we have discussed, a high level of amplification may turn a tiny level of viral particles into the equivalent of an infected patient, which may become a big issue when the initial viral levels of these patients would fall below what would actually be considered an infected individual.
All of these concerns have compounded to create some controversy over the idea of what should be considered presymptomatic, someone who is at the very early stages of the infection and have not presented with symptoms yet, or asymptomatic, as in these people are infected but will never experience symptoms.
Because of the high level of sensitivity PCR tests have a chance of providing a false positive result. I have discussed previously how there really was no way of getting rid of a positive test, even through dilution or long-term storage the sample can still come up positive.
The massive use of PCR without taking into consideration the high sensitivity may have clouded many of our test results, and it has likely contributed to the high number of supposedly asymptomatic cases, a category which we should really question as an actually valid category given the circumstances, and that there really has never been evidence of communicable diseases being spread so widely by those who lack any symptoms.
Even with Antigen tests becoming widely available, PCR tests are still considered the most accurate tests. There’s plenty of good reasons to still rely on PCR, although concerns should still be raised in regards to how high the Ct Cutoff is placed and whether asymptomatic people are actually infectious. It’s all still an issue that we continue to deal with to this day when using PCR tests, and one that’s not likely to be resolved anytime soon.
Antigen Tests- A Brief Overview
Antigen tests have become a lot more widespread in their availability. Unlike PCR tests, which amplify specific genes and retroactively quantifies a patients viral load, an Antigen test specifically tests for the presence of the virus through the detection of viral antigens and their ability to bind to a specific membranous surface and provide a color indicator.
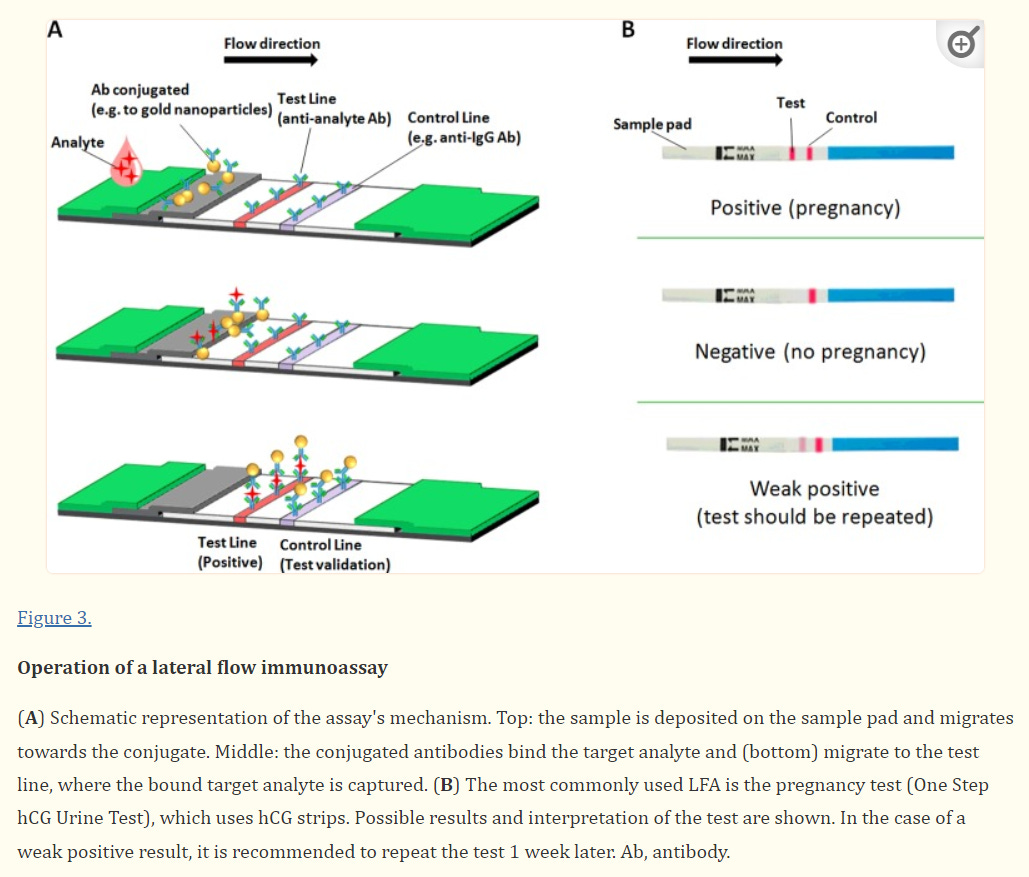
The assay utilized in Antigen tests is referred to as a lateral flow immunosorbent assay. In such an assay, a solution is added to an absorbent paper or membrane with at least 1 region where antibodies are attached to the paper (this is where the “immunosorbent” term comes from). These antibodies target specific analytes within the solution, such as the spike protein (S) or the nucleocapsid (N) protein of SARS-COV2.
The solution then moves through the paper through capillary action, and as it passes through the region of the paper with the antibodies against SARS-COV2, any viral antigens within the solution begin to attach to the antibodies and adhere to that region of the paper. As the number of adsorbed viral antigens increases, a colored stripe appears indicating the presence of SARS-COV2. If no SARS-COV2 is present, or if the antigen load is below the limit of detection no discernible colored stripe may be present.
It’s important to note that many of these tests come with a control line, which is used as an indication for a test’s validity; a cartridge with no control line appearing would suggest that the test is faulty and its results should not be considered valid.

Sample collection for this assay is similar to PCR tests and usually uses nasal swabs that are dipped into an analyte solution, which is then added onto the cartridge in the correct sample region.
If all of this sounds slightly familiar, the technology used in an Antigen test is the exact same as that utilized in a pregnancy test, however in a pregnancy test there are usually two regions of antibodies with one region binding luteinizing hormone and the other binding follicle-stimulating hormone, the two hormones which ramp up in concentration when a women becomes pregnant.
A great benefit of Antigen tests is that they could be done at home and results can be interpreted within several minutes, hence the reason why many of these tests are referred to as rapid tests. However, unlike PCR tests, Antigen tests are not quantitative, such that there’s no way to directly correlate the intensity of the test line to a numerical viral concentration, although heuristically speaking the line’s intensity may be used as a relative gauge. Because of this, most Rapid Antigen tests provide a qualitative result more than a quantitative result, and therefore should not be used as a way of estimating how much actual viral load a person may have.
Antigen tests also don’t have a built-in amplification step, meaning that a high viral load may be required to adsorb onto the cartridge membrane, which unfortunately makes Antigen tests less sensitive due to the high limit of detection. This is also why Antigen tests tend not to be accurate in people who are in the very early stages of the disease such as in the presymptomatic stages, as they are not likely to have a high enough viral load to become detectable. When an Antigen test is deployed within this early time frame of the disease, the lack of high viral load is likely to lead to a false negative, such that someone may not receive a positive result even though they are likely to be infected. This leaves Antigen tests with a shorter window of efficacy, however utilizing them during the presence of symptoms should provide an adequate level of accuracy.
Does the Test Matter?
So unlike a PCR test, Antigen tests aren’t as accurate, yet are still a reliable testing tool to use because of their ease and availability. Although they don’t capture the presymptomatic stages of the disease, they are accurate during the symptomatic stages of the disease where there is likely to be a higher level of detectable virus. Pairing the presence of symptoms with a positive Antigen test should provide strong evidence that someone is infected with SARS-COV2.
One of the main reasons I decided to bring up discussing COVID tests is because of the apparent confusion by many about the apparent “inferiority” of Antigen tests. For example, I have seen instances of people who have gotten positive Antigen tests who were then told to get a PCR test “just to make sure”, which essentially goes against what we have described above. Although Antigen tests are not as accurate, they still provide a reliable measure, especially in patients who are symptomatic. Remember that Antigen tests are likely to skew more towards a false negative rather than a false positive. A test should not do either, because such a test would not be considered reliable in any manner and there would be no way such a test would be granted an EUA or FDA approval. Therefore, less skepticism should be put towards a positive Antigen test, especially when someone does indeed present with symptoms whereas a negative test in a symptomatic patient may require further investigation.
Taken altogether, a positive Antigen test should be considered a good representation, and a valid one at that, that someone is infected with COVID. Unless required by bureaucratic reasons to receive PCR tests, employers should take into consideration positive Antigen tests as a valid measure for their sick employees. More importantly, any test should be paired with the presence of symptoms to fully validate that they are indeed positive. Even now, I have seen people comment that they were told to quarantine for 5 days and stay home unless they were vaccinated and did not have symptoms. It seems that even with the addition of Antigen tests there are still places that are botching public health policies with such incoherent testing procedures.
Where to be Concerned about Testing
To add even more confusion, although more context, I do need to point out an area where both PCR tests and Antigen tests are likely to provide inaccurate results, and that’s likely to occur later into the disease where most people have mostly recovered. This is an area where these tests not only may lack any benefit, but that they may also be detrimental and easily misinterpreted.
So far we have discussed these tests are a method of measuring whether someone is infected, however we never discussed the concept of someone being infectious. They may sound similar, but being infectious has essentially been the driving force for many of our policies.
Being infectious means that someone is able to spread active viral particles to others and thus infect them. It is here that these diagnostic tests are being woefully misinterpreted. We usually don’t discuss whether these tests measure infectivity because there’s a general assumption that people are the most infectious at the beginning of the infection. There’s plenty of evidence from prior viral and bacterial infections to prove that point without requiring further explanations. So when using these tests at the beginning of the infection we never make this distinction because the assumption is already built into the diagnosis and testing.
But the same can’t be said for the end stages of the disease where people are likely to have mostly recovered, even if they still present with some symptoms. Here, people are likely to engage in what’s called viral shedding and may be releasing viral particles. However, here the viral particles are less likely to be infectious, and are far more likely to be inactivated. So what’s the problem with testing at this stage? These tests are designed to detect either viral RNA or antigens, however these tests have no way of detecting the infectious nature of these viral particles. To make such a determination would require that viral isolates be collected from individuals, cultured and checked to see if the virus can still replicate. You can clearly see that such a method would take days, and would hardly be worth the time and effort to conduct such a test in order to report a result that would most likely be irrelevant days after the sample is collected. Because of this, these tests may be doing nothing more than validating the presence of viral particles without being able to determine if these viral particles may infect others. It’s essentially searching for scraps of virus at that point.
Unfortunately, this type of nuance is lost when using these tests late into the disease. If inactivated viral particles remain, it is easy to see how you can still test positive in a good portion of circumstances even if you are not able to infect those around you. Because of this, these tests alone are in no ways a good measure of an actively infectious patient, and by themselves should not be used as a determining factor for someone still remaining ill.
There is a possible way of determining this in PCR tests by waiting 24 hours before testing a recovered patient’s sample, as the 24 hour period may provide enough time to degrade exposed RNA and provide a clearer, true positive result. The same can’t be done with Antigen tests which are based on antigens which are likely to take even longer to degrade.
This is why there have been many instances of people still testing positive weeks after being infected. More care should be taken when examining these results, likely through the use of rationale and sensemaking to determine whether someone is likely to still be infectious weeks after their illness.
To conclude this section, PCR tests are the most likely go-to testing procedure for the rest of the COVID, but Rapid Antigen tests shouldn’t be discounted for their ease and accessibility. Even as Omicron presents as far more mild, understanding and knowing the differences between testing procedures should serve as a benefit for those who seek out such tests.
Note: I should point out that, because Antigen tests depend upon antigen/antibody binding in order to provide a result, there should be many questions as to whether the high rate of spike protein mutations in Omicron is likely to alter its attachment and adsorbption to the test cartridge paper and thus provide an incorrect result.
The Antigen tests that my family used state that they target the Nucleocapsid protein, and in fact many of the tests that I have seen have indicated that they target the N protein rather than the spike protein. It may likely mean that the solution used in these kits contains a lysing buffer to break down the viral particles and expose the N protein of the virus, or the test itself may depend upon free-roaming N protein. Regardless, the N protein tends to remain conserved across many variants, and thus Antigen tests that target the N protein are likely to hold up whereas those that target the spike protein may not fare so well in a post-Omicron COVID landscape.
Personally speaking, for my family our Antigen tests came up positive. Paired with my family’s symptoms, along with nearly everyone around us getting COVID as well, we can infer through various evidence that my family did indeed get COVID.
However, the same can’t be said in other circumstances IF there is a lot of doubt about the effectiveness of Antigen tests for detecting Omicron. It’s a shame that, once again, public health policies may have lagged greatly behind the actual direction of the virus to the point that the tests that are being massively distributed may not be as accurate with Omicron. More evidence and research is required in order to understand if these Antigen tests will indeed hold up, and it may be within a consumer’s best interest to check to see what antigen their tests are designed to target.
(I should also point out that, out of the few Rapid Antigen tests I have examined I have not come across one that used the spike protein as the antigen of choice, but be careful to read the insert to make sure what antigen is being targeted in your at-home test.)
Nonetheless, common sense and preponderance of evidence should be used sans a PCR test. Remember, even if it’s not COVID it may be a good idea to still stay home when sick, and employers should do their best to examine their sick policies in a post-COVID world. If anything, COVID should remind us that, regardless of the illness, we should encourage those who are sick to stay home and get better instead of being forced to work when ill.





I don't understand the obsession we have with testing for this virus. Prior to 2020, we never tested people to see if they had a particular type of virus unless they were sick enough to need serious medical attention.
Excellent article and very timely for me.
Is this correct though?
You say "When an Antigen test is deployed within this early time frame of the disease, the lack of high viral load is likely to lead to a false positive, such that someone may not receive a positive result even though they are likely to be infected."
Don't you mean to say 'likely to lead to a false negative'? i.e. it indicates negative but it isn't really?
Shows you that I read it all and pay attention =;o)