Answers from an Ex COVID Tester: Can a PCR Test Differentiate Between SARS-COV2 and Other Pathogens?
What does a positive PCR test indicate?
Before committing to Substack I worked at a University Science Lab. When the pandemic hit we were shuffled over to perform SARS-COV2 RT-PCR testing. Since then I have seen many questions about PCR testing. Some rational, some a bit over the top. This series is dedicated to answer some of those questions and to help provide some of my personal perspective and information (if applicable).
There are many comments online calling into question the veracity of the PCR tests with many people stating it may not be able to differentiate between a flu, cold, or SARS-COV2 infection.
A typical PCR test will amplify (increase the amount) of a specific gene (genetic material that codes for a protein). When it comes to SARS-COV2 that could mean the spike (S) protein which binds to the ACE II receptor on human cells, the nucleocapsid (N) protein which helps to tightly wind up the virus’ genetic material, or it could target the virus’ structural proteins.
Many viruses have conserved regions of their genome meaning that some gene overlap may occur between different viruses. An easy example would be that SARS-COV and SARS-COV2 both contain a gene for the spike protein.
This would mean that if someone suspected of having SARS-COV2 takes PCR test that only checks for the S gene then it would not be able to differentiate between SARS-COV or SARS-COV2 infection and would not provide an accurate test result.
In order to get around this an accurate PCR test would need to test for multiple genes. This procedure is called a multiplex assay, and it helps to exclude any viruses that may have one gene that overlaps with the pathogen in question. Therefore, if we test 3 genes (typically the S, N, and an open reading frame gene- labelled ORF of SARS-COV2) we are far less likely to have an overlap of 2 or more genes between different pathogens. In this instance, an amplification of the S gene as well as the N gene would indicate a SARS-COV2 infection since it is unlikely that 2 pathogens will share both of these genes.
In fact, here is a sample chart used to interpret a PCR run and how to report the results (taken from Thermofisher’s TaqPath™ COVID-19 Combo Kit and TaqPath™ COVID-19 Combo Kit Advanced Instructions for Use pgs. 107 & 108):
This PCR test kit examines 3 different SARS-COV2 genes in its protocol. As you can see from the chart a positive SARS-COV2 test requires that at least 2 genes come back positive, meaning that it reduces the possibility of dealing with overlapping genes. As a side note, if you ever took a SARS-COV2 PCR test you may have seen a test result like the first table without the action column be reported to you.
And with that we should be able to disabuse the claim that the PCR test is not specific enough to detect SARS-COV2 alone; if a PCR test comes back positive it is certain to be SARS-COV2 (whether or not that means a patient is infectious is a different story).
So that leaves us with a different question that has been raised recently.
On July 21st the CDC published an article titled “Lab Alert: Changes to CDC RT-PCR for SARS-CoV-2 Testing” telling biotech companies about a need to change their PCR tests starting 2022.
The article states:
“In preparation for this change, CDC recommends clinical laboratories and testing sites that have been using the CDC 2019-nCoV RT-PCR assay select and begin their transition to another FDA-authorized COVID-19 test. CDC encourages laboratories to consider adoption of a multiplexed method that can facilitate detection and differentiation of SARS-CoV-2 and influenza viruses.”
This has been taken by those skeptical of the PCR tests as a validation that these tests cannot tell the difference between SARS-COV2 and the flu. However, we have just indicated that not to be the case, and we can verify that by looking at one of these kits.
Here is an excerpt from the “BD SARS-COV-2/Flu for BD MAX System- Instructions for Use” manual on pg. 1 (emphasis mine).
“The BD SARS-CoV-2/Flu for BD MAX™ System utilizes multiplexed primers and probes targeting RNA from the nucleocapsid phosphoprotein gene (N1 and N2 regions) of the SARS-CoV-2 coronavirus, a conserved region of the matrix protein M1 gene for influenza A, conserved regions of the matrix protein M1 gene and hemagglutinin (HA) gene for influenza B, and the human RNase P gene. The primer and probe sets for SARS-CoV-2 are based on the United States Centers for Disease Control and Prevention (US CDC) assay for specific detection of SARS-CoV-2 by amplifying two unique regions of the N gene (i.e., N1 and N2). SARS-CoV-2 targets, N1 and N2, are indistinguishable as they are detected in the same optical channel. Influenza B targets, M1 and HA, are also indistinguishable and are detected in the same optical channel.”
As we can tell from this description, there are specific regions being looked at for these viruses (N1 and N2 regions of the nucleocapsid protein for SARS-COV-2, the matrix protein M1 gene for influenza A, and the matrix protein M1 and hemagglutinin (HA) for influenza B. The matrix protein M1 regions being looked at are unique between influenza A and B but are conserved among different strains of each virus).
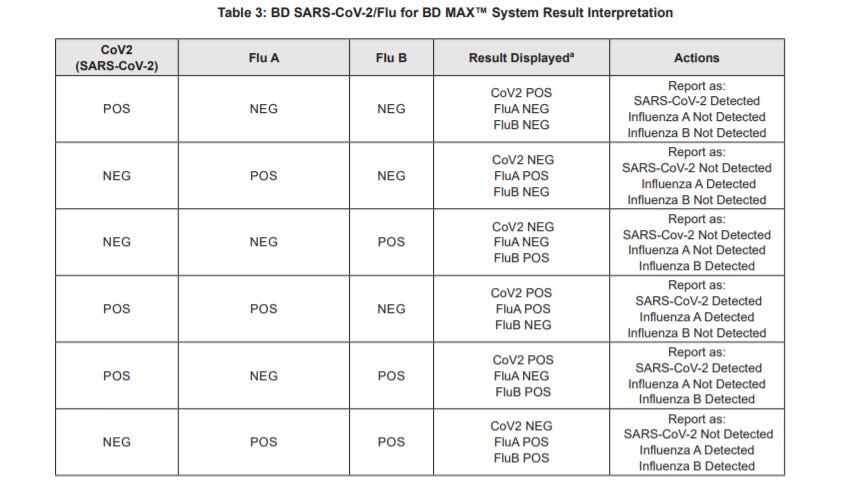
Here’s a reference interpretation chart for this PCR kit pgs. 8 & 9:
So if we are still able to differentiate between a SARS-COV2 infection and a flu infection how do we interpret the lab alert put out by the CDC? It seems instead that the CDC is alluding to the idea that SARS-COV2 will become (or is) endemic, and similar to the flu will have seasonal spikes. This would mean a multiplex assay that tests for both SARS-COV2 and the flu would be easier, cheaper, and quicker to conduct than several separate PCR tests.
There have been many discussions about the feasibility of eradicating SARS-COV2 from the population, and whether or not this is the CDC “admitting” that COVID-19 will be with us from here on out, it does seem likely that we will continue to see SARS-COV2 infections in the foreseeable future.
If there are any questions about COVID-19 testing you would like answered please leave a comment below (this newsletter was made available to comment by anyone including non paying subscribers).
Thank you for reading my newsletter. If you enjoy my articles please consider becoming a free subscriber in order to receive notifications.
And share with others who may find these newsletters interesting.
Also, please consider becoming a paid member. The research and work put into these articles takes many hours and being a paid subscriber allows me to continue to do this full time.





I really appreciate this sort of clarity. I was taken in for a bit by reports that the CDC was admitting that their tests couldn't differentiate between the flu and COVID and thought that was why flu cases disappeared almost entirely last year: the CDC was just counting them all as COVID. After re-reading the passage, however, I could see that it didn't exactly say what I prima facie thought it did and I went to the FDA website for answers regarding the specificity of their texts. I found the assays there, but couldn't answer the question. I've been wondering ever since, so thanks for clearing this up! Now, though... where did all the flu go last year? And why does the CDC still report that flu and pneumonia are by far the most common comorbid conditions of those who died with/from COVID?