Nothing signals the coming of the fall season more than the flurry of warm colors that, ironically, signal the end of the warm season themselves1.
Even during Labor Day weekend as I was grilling outdoors I noticed many leaves already falling, cascading through the air and imbibing it with hues of yellow from nearby trees.
Reds, yellows, and oranges make up the color scheme for much of this time of year within the Northern hemisphere, and yet we may not take the time to notice that only certain trees may change leaves.
Why is it that some leaves only turn yellow while others may only turn red?
So where exactly do these colors come from, and why do they change during the fall season?
We’ll explore some of these ideas and more in this post.
Chlorophyll me up
Before discussing the changing colors it may be necessary to explain the most common leaf color of all- green.
Most plants in temperate climates contain green leaves. Similar to money green for most plants is a symbol of energy and power. It provides plants with one of their most important biochemical reactions: photosynthesis— a biochemical reaction that captures light, water, and carbon dioxide and uses that energy to create carbohydrates and energy for the plant while also releasing oxygen for us.
In most biology classes students are taught that the green color that comprises most plants comes from the molecule Chlorophyll.
Chlorophyll is a pigment molecule with a backbone analogous to the molecule Chlorin2 and generally comes in two forms within plants- Chlorophyll a and Chlorophyll b.
Yes, they’re both not very clever names, and it’s made worse that both molecules are highly similar with one of the only notable differences being the aldehyde functional group found in Chlorophyll b that is missing in a (circled below).

A biochemical discussion of photosynthesis will be outside the intended scope of this post (maybe I’ll save it for spring), but in essence Chlorophyll molecules with a Magnesium-chelated ion capture energy from the sun's photons--particles emitted by the sun that contain energy. The photons excite Chlorophyll and move one of the electrons to an excited state3 (a higher orbital). From there, that energy contained within the excited electron may be passed between other Chlorophyll molecules until they reach the necessary reaction center where photosynthesis occurs.
However, the excited electron may move back down to its original orbital and release the photon and fluoresce. It’s this released photon and fluorescence that provides the color we perceive.4
Therefore, pigment molecules get their color not from the color that they absorb, but the color that they reflect. It is the wavelength that is reflected that we perceive with our eyes.
For Chlorophyll a and b both molecules absorb light5 from the blue and red/orange spectra of visible light6 and don’t absorb any green light- hence why many leaves appear green.
The following absorption spectra shows Chlorophyll a and b as well as Chlorophyll d and f which are found in cyanobacteria. Note the valley between 500-550 nm— the range for green light, which indicates that this color is reflected rather than absorbed by Chlorophyll.

In short, the color green is vital to a plant’s ability to produce energy. Chlorophyll’s ubiquitous nature highlights an evolutionary history and its role in providing necessary energy to plants in the form of photosynthesis.
But that’s not why we’re here— we can talk more about green leaves come spring.
Now that we’ve set up where green leaves come from let’s explore where the orange, yellow, red, and brown hues of fall get their colors.
Autumn leaves and the mechanisms of color change
Now let’s talk about where the colors of autumn leaves come from. Similar to Chlorophyll and green leaves, the colors found among fall leaves are derived from various compounds.
A helpful guide can be seen below. It spoils a bit of the surprise, but we can manage given how informative and succinct the image is. Note the different compounds that contribute to the different colors seen:
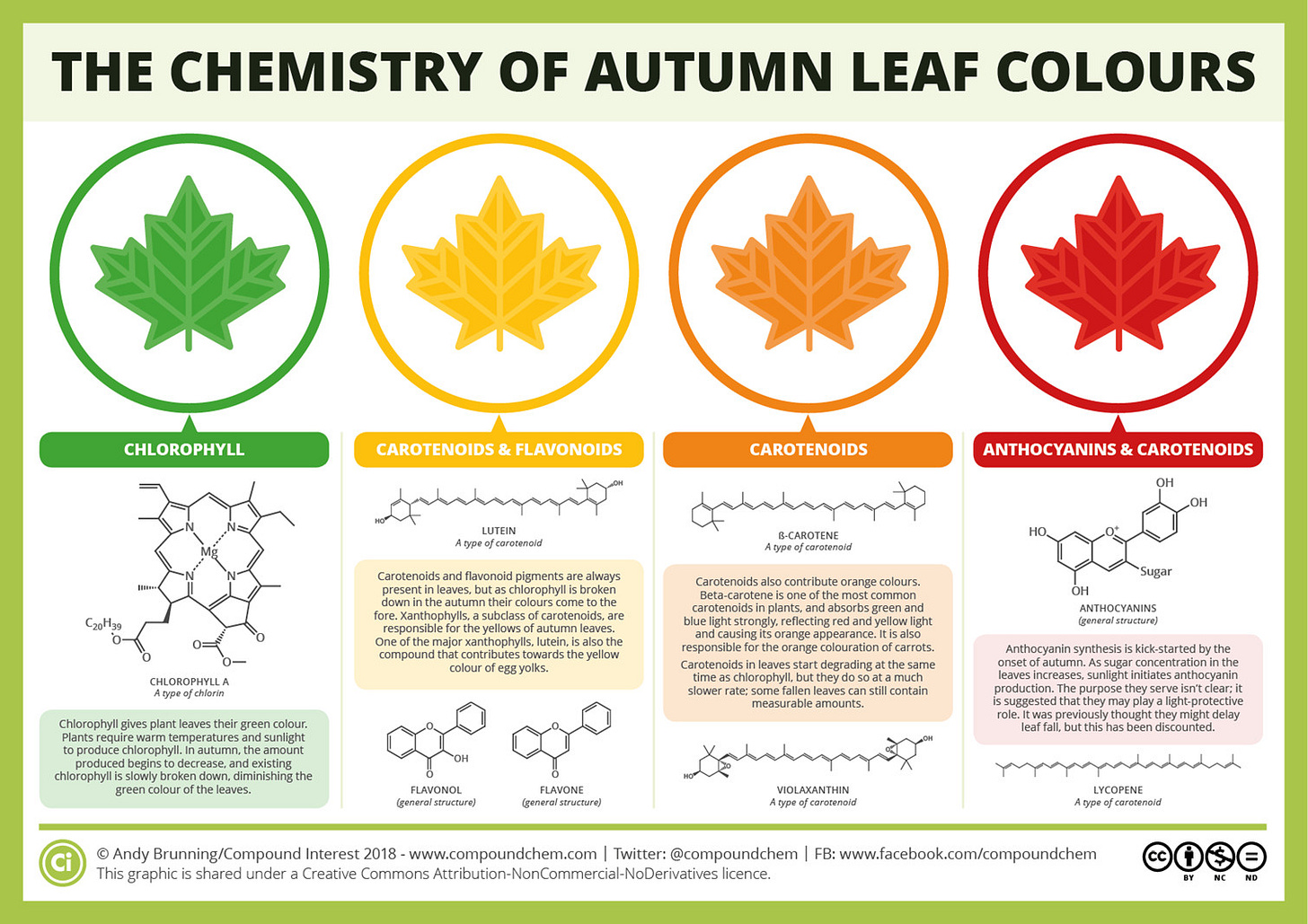
This descriptive review from Archetti, et. al.7 is also handy if the above text is too small:
Although a few classes of molecules contribute to the overall color of leaves, it’s the Carotenoids and the Anthocyanins that are the most important and will be discussed here.8
Carotenoids- bringer of yellow and orange
Carotenoids are one of the most common class of pigments, comprised of a hydrocarbon, lipid-soluble chain and two end ring structures. Carotenoids provide yellow, orange, and red hues to fruits and vegetables with the most common being Beta-carotene9 found in carrots and Lutein found in egg yolks.
The presence of Carotenoids in plant leaves appear to provide both a photoprotective and antioxidant effect— all due to, of course, the high degree of resonance from the hydrocarbon chain comprised of alkenes named a polyenic chain10 (essentially many alkenes).
Carotenoids can scavenge for free radicals and act as a sacrificial molecule to mop up reactive oxygen species and other free radical agents. The formation of a Carotenoid radical can then be stabilized due to the shuffling of the radical around the molecule or conjugation of one Carotenoid radical with another which can then be reduced or removed.
This ability to scavenge for ROS and other free radicals has led to extensive research into the use of Carotenoids for cancer and cardiovascular disease, and the photoprotective effects have looked into Carotenoids as possible therapeutic agents for photo-related disorders.11
The presence of Carotenoids in leaves are assumed to protect the leaves from oxidative damage that may disrupt photosynthesis.
And if you were curious, yes eating too many carrots can lead to high levels of circulating beta-carotene and the condition called carotenemia in which the skin may appear discolored and orange. However, most images of carotenemia aren’t dramatic or obvious, so don’t expect to find pictures such as Arnold from the Magic School Bus floating around for real people.12
So if Carotenoids are present at the same time as Chlorophyll why is it that leaves aren’t a weird mix of yellow-green (or green-yellow?)?
Supposedly, the green color from Chlorophyll masks the colors from Carotenoids. However, as the leaf begins to die (senescence) in autumn the Chlorophyll decomposes and the Carotenoids begin to shine through.
Archetti, et. al. provides this insight [context added]:
Although carotenoids are present all year round in the leaves, they are masked in mature leaves by the green of chlorophyll; in autumn, they become visible because of the breakdown of chlorophyll into colourless metabolites, but there is no evidence for a de novo synthesis [there doesn’t appear to be evidence of Carotenoid production as the leaf undergoes senescence] [9].
An overlap of an absorption spectra comparing Chlorophyll a and b to Carotenoids shows that Carotenoids absorb within the violet-blue spectrum similar to Chlorophyll. However, unlike Chlorophyll Carotenoids don’t absorb within the yellow-red spectrum. Therefore, as Chlorophyll breaks down less yellow-red light can be absorbed, more is reflected and we are able to see more of those colors.

So if Carotenoids and Chlorophyll are all present on a healthy, green leaf at the same time why is it that Chlorophyll breaks down while Carotenoids may remain?
The first chart for this section suggests that Carotenoids may break down slower than Chlorophyll thus having an overall longer stay, but that is a rather ambiguous statement.
Instead, it could be due to the location of Chlorophyll that may infer some underlying mechanism. Chlorophyll is located within the chloroplasts of plants. The biochemical processes that occur within chloroplasts are very similar to those seen in mitochondria, and just like mitochondria the chloroplast is capable of producing many ROS that may damage plant cells and the nearby environment.
Under times of intracellular oxidative stress or stress from the environment chloroplasts may undergo chloroplast dismantling: a tightly controlled process in which chloroplasts are broken down, Chlorophyll is metabolized, and amino acids and vital elements such as nitrogen and carbon are shuffled to sink tissues to aid in plant growth.
The organized breakdown is carried out to limit the detrimental effects of free radical release that may cause oxidative damage to nearby organelles and cells. Essentially, it’s a contained oxidative explosion.
Domínguez, F. & Cejudo, F. J.13 provide this in the conclusion of their review. The review is rather detailed so take a gander if you are interested:
Moreover, chloroplasts have the important activity of sensing external environmental conditions that allows the harmonization of the growth of the different plant organs. Nevertheless, stressful environmental conditions may imbalance ROS production and scavenging, which generates oxidative stress and potentially may cause damage to the organelle. These conditions trigger mechanisms of chloroplast degradation, which have the function of avoiding the deleterious effects of damaged chloroplasts on cell viability. In addition, leaf senescence is a complex genetic programme that allows the remobilization and recycling of leaf components to support the growth of sink tissues, and chloroplasts constitute an important source of carbon and nitrogen in this recycling process. Therefore, chloroplast dismantling is an essential process of plant development and adaptation to stressful environmental conditions.
This same process does appear to contain mechanisms to metabolize Carotenoids, but to the extent that this may occur is, again, ambiguous.
In some ways this process of chloroplast dismantling makes sense as many leaves change color before falling off the tree— green leaves may be indicative of wasted nutrients as the tree doesn’t have a viable way to reuptake those amino acids, carbon, and nitrogen if they fall off beforehand.
And so an organized takedown of chloroplasts during leaf senescence may lead to the yellows and oranges that we see. It’s a reminder of how some of the most aesthetically pleasing offerings from nature may be masked until the right time. As a tree stores energy for coming winter months, it provides us with that hidden gem that only comes at this time of the year.
Anthocyanins- the dark maker
This title is a bit of a misnomer, but I also couldn’t think of another appropriate title 🤷♂️
The next pigment molecule are the Anthocyanins which contribute to the red leaf color.
Anthocyanins are a class of flavonoids conjugated to sugar molecules and are found in many plants and berries. They are vacuolar pigments (found in the vacuole of plants) and their color is pH-dependent. In acidic environments they take on red hues while in more basic environments they take on blues and violets.

If you ever used red cabbage as a pH indicator as a child, you were most likely looking at the color change of the Anthocyanins.14
The name Anthocyanin is actually Greek with ánthos meaning “flower” and kuáneos (kyanos) meaning “dark blue”. This name is fitting, as darker plants generally signify an abundance of Anthocyanins:
Because of the pH dependent nature of Anthocyanins absorption spectra may stratify absorption based on the pH of the solution.
One example is this study from Ahmadiani, et. al.15 in which researchers isolated Anthocyanins such as cyanidin-3-diglucoside-5-glucoside16 (Cy3diG-5-G) from red cabbage and compared absorption spectra across different pH values:

Strangely enough, the pH-dependent activity of Anthocyanins can be used to infer the pH of given plant structures.
Since Anthocyanin-rich tree leaves are red, that tells us that the environment of these leave’s vacuoles are likely to be acidic as can be seen in the absorption spectra at a pH of 2 (bold line) and 5 (small, dashed-lines) which shows no absorption at higher wavelengths at these pH values.
The history of red leaves is a rather interesting one, as original work into the red color of autumn leaves made assumptions similar to those for yellows and oranges, in that they may be exposed as Chlorophyll begins to break down.
Take this history lesson from Lee, D. & Gould, K.17:
Interest in the red coloration of leaves and other plant parts can be traced back to the beginnings of western science. Red coloration in leaves was observed by Aristotle (Barnes, 1984) in his writings on the theory of colour, and by Theophrastus (1918) in his descriptions of different plants. During the renaissance, Nehemiah Grew (1682) observed the red pigmentation in vegetative organs and conducted simple experiments on them, described in his "The Anatomy of Plants". Various scientists observed the distribution of 'coloured cell sap' in plant organs with the light microscope, for which Marquart (1835) coined the term anthocyanin, derived from the Greek anthos (flower) and kyanos (blue). Many long-standing misconceptions on anthocyanin function were derived from these early observations. For instance von Mohl (1837) held that anthocyanins were derived from the breakdown of chlorophyll, based on his observations of autumn leaf coloration. Clearly, this is incorrect, yet the adjunct hypothesis, that anthocyanins are revealed during leaf senescence as chlorophylls degrade (which is also incorrect) persists in the contemporary literature.
However, given the fact that many plants actually present with red leaves, such as plants found within tropical climates, another reason for this color had to be found. In fact, it’s the examination of red tropical plants that warranted other reasons for the emergence of red.
During this time [early 20th century], work on anatomy, morphology and physiology in plants became fused as a new discipline: physiological plant anatomy (Haberlandt, 1914; Cittadino, 1990). Much of the inspiration for this research was the experience of Eurepean botanists in the tropics, particularly in Southeast Asia. There, they observed the presence of red pigmentation in tropical plants, particularly the rapidly expanding foliage of trees and the undersurfaces of shade plants. The striking coloration in leaves of these plants, rarely observed in temperate regions where speculation was primarily derived from observations of autumn leaf colour, stimulated much research on anthocyanin function in leaves.
But what’s peculiar about the presence of Anthocyanin within red tree leaves is the fact that these compounds appear to be produced de novo; that is, they are produced by the leaf as senescence is underway.
Archetti, et. al. provides this little comment:
Anthocyanins, by contrast, are newly generated in autumn, shortly before leaf fall [10–13]. Thus, red is produced actively in autumn and is not simply the side effect of leaf senescence.
This is really strange, as unlike the yellows and oranges from Carotenoids that are already dispersed within the leaf, this would indicate that there may be an underlying reason for the production of these Anthocyanins.
So why exactly would a leaf produce new compounds at the time that it is dying?
This has led many plant evolutionary biologists and ecologists to wonder if there is an evolutionary basis for the red leaves, questioning if there is an adaptive reason for this strange phenomenon:
What use is the production of a red pigment in leaves that are about to be shed? How do we explain the interspecific variation, or the fact that only some species turn red in autumn?
There are two kinds of adaptive explanation for the function of red pigments in autumn leaves: (i) protection against abiotic factors, and (ii) animal–plant interactions. The possible abiotic functions of anthocyanins reduce to three hypotheses [12,18]: photoprotection, osmotic regulation and warming. Many additional possible functions have been proposed that rely on an interaction between plants and animals [18]: coevolution, fruit flag, direct defence, camouflage, anticamouflage and tritrophic mutualism. Only the photoprotection hypothesis [2–4] and the coevolution hypothesis [6,7] have been tested recently, and here we describe recent evidence for both. The other hypotheses (Box 3) have either been dismissed, remain untested or can be reduced to other previous hypotheses [18].
I’ll include just a few hypotheses here, just know there are a ton floating around. More can be found in the Lee, D. & Gould, K. review for those interested.
1. Photoprotective effects from Anthocyanins
One of these hypotheses suggests that Anthocyanins may provide a photoprotective effect during the fall season. As it gets colder the sun causes greater photo-oxidative damage to leaves. Remember that it’s not the leaves itself that are important, but the nutrients within the leaves that are taken back up into the tree (called resorption), and so Anthocyanins may provide a buffer as nutrients return back to the tree and reduces the number of “damaged nutrients” so to speak:
The risk of photo-oxidative damage is especially high in autumn, because (i) cold temperatures reduce carbon fixation capacity; (ii) there is increased light owing to a thinning canopy, affecting shade-adapted, understory trees; and (iii) there is decreased self-shading by chlorophyll as breakdown occurs [20]. The adaptive function of photoprotection would not be the protection of leaves per se (they are going to fall shortly anyway), but that functional leaves enable a better resorption of nutrients, especially nitrogen and phosphorus [3,4].
2. Coevolution with insects to avoid predation and parasitism of leaves
One fascinating hypothesis is that the red color may actually dissuade insects from laying siege and parasitizing the leaves of trees, as well as the tree itself. As such, the red color may serve as visual cues to insects that the leaves are not a hospitable environment, hopefully dissuading them from laying eggs and damaging the plant:
According to the coevolution hypothesis, autumn colouration is a signal of quality directed to insects that migrate to the trees in autumn: red might be a signal that the tree is not a suitable host for insects, because of high levels of chemical defences, lower nutritional quality or imminent leaf fall, or any other characteristic that would induce a lower fitness in the insects [6,7,38,39]. Possible receivers of the signal are insect species that migrate to the trees in autumn. Many aphid species, for example [40], migrate from their summer host (usually a herbaceous plant) to trees in autumn: they land on the leaves and lay their eggs on the trees’ twigs, often close to the winter buds; the eggs hatch in spring, when aphids develop on the tree before migrating to their summer host.
Because autumn migration is a crucial step in the life cycle of many insects [40], they are under strong selective pressure to find the most suitable host, and because many insects respond to colours they could use leaf colour as a signal of the quality of the tree. The tree, by contrast, would benefit from reducing its insect load, because insects can cause considerable damage to their host trees, particularly in spring, when the next generation hatches from the eggs laid in autumn. Insects cause damage not only through direct feeding but also because they are vectors of viruses, pathogenic fungi and bacteria. Insects moving to the trees in autumn would preferentially colonise green rather than red leaves, and trees with red leaves would reduce their insect load. Autumn colours and the preference of insects for green leaves would therefore coevolve in an arms race: red leaves as an adaptation to reduce insect-induced fitness costs, and insect preference for green leaves as an adaptation to find the most suitable host trees.
Again, all of this is an extremely costly endeavor. Production of Anthocyanins would require upregulation of the necessary genes, production of the proper enzymes and then the necessary control of the needed biochemical processes.
Plants may not do so unless the cost of not doing so would be devastating. In essence, production of these Anthocyanins may be an insurance policy taken out by plants to reduce more costly consequences.
3. Protection from oxidative stress and ROS.
Similar to Carotenoids, one hypothesis suggests that Anthocyanins may provide antioxidant properties and attenuate the risk of oxidative stress (Lee, D. & Gould, K.):
Anthocyanins may mitigate oxidative damage in leaves subjected to biotic or abiotic stress (Yamasaki, 1997; Neill et al., 2002). Anthocyanins scavenge most species of reactive oxygen (Bors et al., 1994). They could also reduce photooxidative stress by reducing the light flux incident on chloroplasts (Neill, 2002), and by chelating transition metals in the cell vacuole (van Acker et al., 1996).
Anthocyanins have been extensively researched for their antioxidant properties and possible therapeutic effects for inflammation. They’ve also been looked at as a natural source of food coloring.18
One caveat to this argument is that other free radical scavenging molecules are available, and so one has to wonder whether the cost of investment for something many other molecules are already capable of may be a viable use of energy. It could be that scavenging for free radicals may be an added benefit, but further research may provide additional reasons.
And so the color of red, autumn leaves may not be a consequence of being hidden behind the greenery, but may be an evolutionary advantage that provides some benefit (albeit costly) to plants that may provide new defenses during the fall months.
Scientists continue to find new hypotheses for this red color change, and even now some of these hypotheses have not been fully elucidated.
As mentioned in some of these review articles, this may be a consequence of lack of communication between disciplines. When Anthocyanins were found to be produced in response to intense light, most research looked more into what was occurring rather than finding out why a plant would have such a response in the first place (i.e. why even produce the red pigments?).
This may be a reason why hypotheses are still running abound without clear answers, as it’s only within the past few years in which researchers began to look for reasons for this color (Lee, D. & Gould, K.):
Early observations that anthocyanins in leaves were inducible by light led to an expansion in research into plant photobiology. The pathway of anthocyanin biosynthesis, with the induction of enzymes at different points, has became a model system in photobiology (Mancinelli, 1985; Beggs and Wellman, 1994) and plant molecular biology (Westhoff, 1998), and is a key component of research on phytochrome (Sage, 1992). Thus, it is not surprising that we have a detailed knowledge of the biosynthesis of these pigments, including the molecular genetics of their control at different points in their biosynthetic pathway. In contrast, we have relatively little knowledge of anthocyanin function(s) in plant organs, other than their potential to attract animals for pollination and seed dispersal.
Of all the colors, red appears to be one that comes with quite the enigma. The diverse array of red plants worldwide raises questions as to what function this red pigment may have. As such, take some time to appreciate the reddening of leaves and think to yourself what may be happening.
Do a little investigating, and come up with some of your own ideas. This weekend consider wandering around and see whether red leaves attract insects, or if green is the favored color. Maybe even observe a leaf and look for color splotches. Is there orange and yellow mixed in with a predominately green leaf? Maybe chlorophyll molecules are being degraded.
Overall, take the time to examine your surroundings. Ask yourself why some things are the way they are. Be curious, be a bit adventurous, and see what you can find if you spend some time outdoors.
Additional context worth considering
Before ending this post here’s some additional things worth considering while wandering outdoors.
Which tree leaves give off which colors?
The US Forest Service provides some information on specific trees and which colors their leaves turn:

Archetti, et. al. also has some information about certain tree species:
Red autumn colours are present in 10% of the tree species of the temperate regions, whereas yellow is present in 15% of the species [14]. In some regions of the world these frequencies are much higher, for example in the mixed forests of New England in the USA, where 70% of woody species turn red and 30% yellow [5], and in the southern beech forests of Patagonia, where pure forests of Nothofagus become orange and red in autumn (Figure 1).
There is an extensive phylogenetic variation, with 50% of maple (Acer) species turning red, and entire taxa with no autumn colours [14].
What causes red, non-autumnal leaves?
Although this review included references to red tropical plants it did not go deeper into the topic as to why certain plant species may remain red all year.
Archetti, et. al. does have this comment about a few species of plants. This may have to do for now, and in the future I may consider a deeper dive into red tropical plants:
If there are any questions please feel free to ask! But for now, considering taking this information and do your own investigation this weekend. Apply this information and let us know what you may find!
If you enjoyed this post and other works please consider supporting me through a paid Substack subscription or through my Ko-fi. Any bit helps, and it encourages independent creators and journalists outside the mainstream.

I’m, of course, leaving out everything that has been laced with pumpkin-spiced whatever.
Chlorin has the following structure:
You may have noticed that the Chlorin backbone is seen in another molecule besides Chlorophyll: Heme which acts as a cofactor for the protein hemoglobin and helps transfer Oxygen around by forming a complex with the heme iron cation (rather than a magnesium cation):
Heme is also an important cofactor for the electron-transport chain (ETC) where the iron cation undergoes oxidation/reduction to carry electrons that drive the formation of ATP. Okay, enough Biochemistry! But it’s interesting to see that the similar Chlorin is seen across various biochemical processes.
Why can Chlorophyll stabilize the excited electron? That’s right, resonance! I’m sorry…
For those who may be interested in the fate of the photon that hits Chlorophyll, here are some of the following pathways.
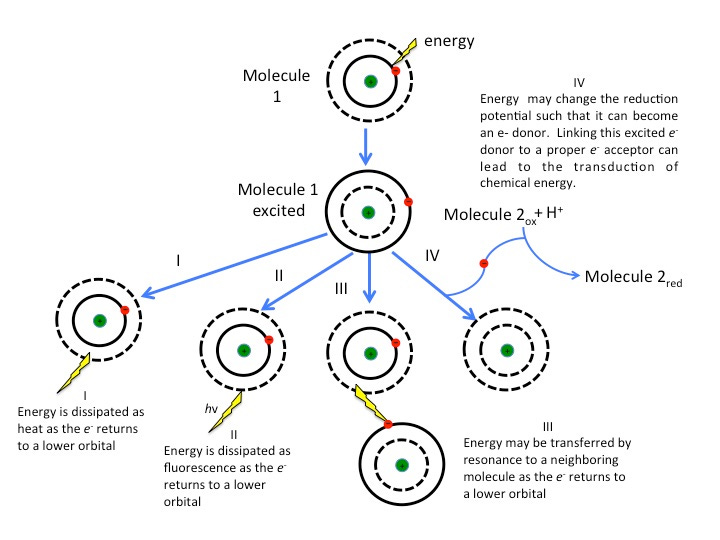
These measures generally come from a method called spectrophotometry, in which light passes through a substrate and absorbance is measured based on transmission or reflection of light as measured by a spectrophotometer. The results provide a spectrum called an absorption spectrum in which the peaks indicate which colors are being absorbed and valleys show the colors that are reflected.
The visible light spectrum is a portion of the electromagnetic spectrum that we can see (i.e. visible), usually within the range of 700 nm (red) - 400 nm (violet). Energy and wavelengths are inverses of one another, such that the longer the wavelength the less energy it contains. That’s why UV light tends to be more dangerous than infrared light.
The following spectrum is organized by longest to shortest wavelength going from left-to-right.
Archetti, M., Döring, T. F., Hagen, S. B., Hughes, N. M., Leather, S. R., Lee, D. W., Lev-Yadun, S., Manetas, Y., Ougham, H. J., Schaberg, P. G., & Thomas, H. (2009). Unravelling the evolution of autumn colours: an interdisciplinary approach. Trends in ecology & evolution, 24(3), 166–173. https://doi.org/10.1016/j.tree.2008.10.006
Several other pigment molecules contribute to the fall colors, but since descriptions of all of these other colors would make this post exhaustive I have limited it to the main ones.
In the figure for Footnote 8 the polyenic chain is boxed in yellow. Note that this structure is comprised of many alkenes and creates a highly conjugated resonance system that contributes to the color of plants and foods that contain this molecule.
Fiedor, J., & Burda, K. (2014). Potential role of carotenoids as antioxidants in human health and disease. Nutrients, 6(2), 466–488. https://doi.org/10.3390/nu6020466
I had to look up and remember why Arnold turned orange. He apparently ate a ton of Sea Wheedies (the show’s Goldfish crackers) causing him to turn extremely orange.
Fernando Domínguez, Francisco Javier Cejudo, Chloroplast dismantling in leaf senescence, Journal of Experimental Botany, Volume 72, Issue 16, 11 August 2021, Pages 5905–5918, https://doi.org/10.1093/jxb/erab200
If anyone wants to try this experiment at home here’s an interesting outline and imagery of that process. It’s hard to see, but note that any tiny circle next to Oxygens denote protonated Oxygens. As the pH increases (becomes more basic/alkaline) the color lightens until highly basic environments in which one of the rings splits due to a base-catalyzed reaction.

Ahmadiani, N., Robbins, R. J., Collins, T. M., & Giusti, M. M. (2016). Molar absorptivity (ε) and spectral characteristics of cyanidin-based anthocyanins from red cabbage. Food chemistry, 197(Pt A), 900–906. https://doi.org/10.1016/j.foodchem.2015.11.032
Lee, David & Gould, Kevin. (2002). Anthocyanins in leaves and other vegetative organs: An introduction. Advances in Botanical Research - ADVAN BOTAN RES. 37. 1-16. 10.1016/S0065-2296(02)37040-X.
Alappat, B., & Alappat, J. (2020). Anthocyanin Pigments: Beyond Aesthetics. Molecules (Basel, Switzerland), 25(23), 5500. https://doi.org/10.3390/molecules25235500













Autumn colors are why I would make an horrible biologist.
Instead of delving into the nuances of cholorophyll and all the rest, I'm far more interested in taking in the majesty and poetry of the annual fall tapestry.
And yet the knowledge that there is a discernible and discoverable science behind that tapestry only adds to the sheer wonder that is this divine creation we call Earth.
(Now, why is the answer to life, the universe, and everything 42? :D )
Nicely presented. I found myself consolidating a number of different traditions while reading the article. It’s important that we are reminded that events are not random nor are they generally singular and unrelated.
I have notice through several decades that my sugar maples product different Fall colors depending on the heat and rainfall of the Summer. Thanks, I enjoyed the intellectual stimulation and break from the broken immune systems and illness. Although the article did relate to nutrition and free radicals. Everything comes full circle.