An Introduction to Exercise Immunology
Examining the relationship between the immune system and exercise.
In the last post we covered how exercise provides a host of different benefits to us. Here, we’ll dive into some information and see what may be happening with the immune system when we exercise, and whether these events are beneficial are harmful.
As you can tell by now, exercise may cause wear and tear on the body, and repair of the body may lead to inflammation. That should indicate to us that something may be happening (albeit superficially) immunological-wise when we exercise to either elicit inflammation or dampen it.
In the past few decades a field of immunology has emerged examining the relationship between exercise and the immune system called exercise immunology. It’s through much of this research that scientists have looked at the intrinsic relationship between exercise and the immune system, whether by measuring acute (immediate) effects on the body through exercise or whether chronic, long-term forms of exercise may cause permanent changes to our immune system.
Much of this may come from the physical stress caused during exercise, and may scientists may use exercise as a model for other forms of traumatic stress due to overlaps.
This is explained in a review from Pedersen, B. K., & Hoffman-Goetz, L.1, and it’s a review I’ll be drawing from quite often for this post:
Over the past 15 years a variety of studies have demonstrated that exercise induces considerable physiological change in the immune system. The interactions between exercise stress and the immune system provide a unique opportunity to link basic and clinical physiology and to evaluate the role of underlying stress and immunophysiological mechanisms. It has been suggested that exercise represents a quantifiable model of physical stress (113). Many clinical physical stressors (e.g., surgery, trauma, burn, and sepsis) induce a pattern of hormonal and immunological responses that have similarities to that of exercise. Whereas neural-endocrine-immune interactions have been investigated using a variety of psychological models (176), the exercise model provides a further opportunity to establish these links using a physical stress paradigm.
On the surface this may sound a little alarming- why would exercise be used to measure other forms of trauma? Wouldn’t that indicate that something bad may be happening when we exercise?
To answer such a question requires a bit of nuance, and it requires us to understand what exactly is going on in the body while we exercise.
Most of this information may be a bit too technical, so I will try to summarize it in an approachable manner. Also, as we will cover below, there’s a lot of controversial findings within the field of exercise immunology, which may not be substantiated or based on flawed studies. We’ll cover more of that further on.
Immunological Changes from Exercise
Many studies on exercise immunology have looked at acute changes within the body. Acute changes generally refer to immunological changes during exercise or within the first few hours after exercise is completed. For the sake of this post, most of the evidence here will pertain to acute studies, as the number of long-term studies relating immunological changes are very limited.
In general, many immune cells are deployed during exercise. This includes lymphocytes, neutrophils, natural killer cells, and many signaling molecules. However, there are a few cells that appear to decline in the aftermath of exercise.

Now, there’s a ton of stuff to go through here, and figuring out the reason for each individual cell type being deployed would be beyond the scope of this article. Instead, we’ll go over a few specific reasons for these immunological responses. Additional information can be found in the review articles cited in this post for those who want additional explanations.
As noted above, exercise and tear on the body itself is likely to lead to upregulation and deployment of immune cells, similar to a response seen during an infection (Pedersen, B. K., & Hoffman-Goetz, L.):
The local response to an infection or tissue injury involves the production of cytokines that are released at the site of inflammation. These cytokines facilitate an influx of lymphocytes, neutrophils, monocytes, and other cells, and these cells participate in the clearance of the antigen and the healing of the tissue. The local inflamma-tory response is accompanied by a systemic response known as the acute phase response. This response in-cludes the production of a large number of hepatocyte-derived acute phase proteins, such as C-reactive protein(CRP),a2-macroglobulin, and transferrin. Injection of TNF-a, IL-1b, and IL-6 into laboratory animals or humans(55) will produce most, if not all, aspects of the acute phase response.
And so this overall response may be a response from the body to start recovery and repair, which would be nothing out of the ordinary.
Monocytes and Exercise
For example, monocytes may be deployed after exercise in order to aid in tissue repair (Peake, et. al.2):
Monocytes mobilized by exercise are likely to infiltrate skeletal muscle and differentiate into tissue-resident macrophages that facilitate repair and regeneration, particularly following arduous bouts of exercise that cause significant skeletal muscle damage (85). Monocytes with effector phenotypes are also preferentially redeployed after exercise. The CD14+/CD16+ “proinflammatory” monocytes are preferentially mobilized over their CD14+/CD16− counterparts (109).
Lymphocytes and Exercise
The explanation for lymphocytes, and T-cells in particular, is rather strange and ambiguous. Some researchers suspect that deployment of lymphocytes after exercise is done as a form of immunosurveillance, and may be a method of halting the emergence of latent viruses (Peake, et. al.):
Exercise appears to “prime” effector T cells, thereby allowing them to transmigrate to the peripheral tissues that require enhanced immune surveillance following physical stress (54). Compared with the resting condition, the percentage of circulating lymphocytes expressing effector cytokines is lower following prolonged exercise (115), but it is unknown whether this decline reflects impairment at the individual cell level or preferential movement of effector T cells into peripheral tissues (e.g., lungs and gut). Recent evidence showing that exercise redeploys T cells that are specific to latent herpesviruses such as cytomegalovirus (CMV) and Epstein-Barr virus (EBV; 111, 112) suggests that this response may be a countermeasure against stress-induced viral reactivation (107).
Some researchers suspect that deployment of activated T-cells may make room for new, naïve T-cells by virtue of leading to lymphocyte apoptosis. Originally, this idea was not considered one of merit (Pedersen, B. K., & Hoffman-Goetz, L.):
Thus the initial increase in CD4+ and CD8+ cells after exercise appears not to be due to repopulation by newly generated cells but may be a redistribution of activated cells, in agreement with kinetics of CD4+ lymphocyte repopulation after anti-human immunodeficiency virus (HIV) treatment (147), chemotherapy (96), and CD4+ and CD8+ lymphocyte repopulation after bone marrow transplantation (15).
However, recent evidence suggests that exercise may attenuate senescence (aging) and exhaustive T-cells.
One systematic review from Donovan, et. al.3 examined studies within the literature to see the effects of exercise on the expression of surface markers that indicate senescent or exhaustive T-cells, with an explanation of the difference between the two below:
The terminology and definitions for senescent and exhausted CD8+ T cells are yet to be finalized resulting in a lack of clarity in research for these cell types. In the past, senescent cells have been referred to as late-stage, terminally, or highly differentiated T cells (Turner, 2016; Duggal et al., 2019). Adding to the confusion, the terms “exhausted” and “senescent” T cells have been used interchangeably (Bigley et al., 2013; Pawelec, 2019). Exhausted and senescent T cells have different origins, identifications (protein surface markers), and functional ability. Exhausted T cells arise from excessive and prolonged stimulation of the T cell receptor (TCR) and the action of inflammatory cytokines, which progressively suppress T cell effector functions (Pawelec, 2019). In contrast, senescent T cells arise through aging and/or chronic infection (Turner, 2016).
With a Table providing detailed explanation for individual surface markers and what they indicate:
Based on their review, Donovan, et. al. suggest that various exercise routines may reduce senescence and exhaustion of CD8+ T cells, although the literature continues to be limited in their findings to fully support such a position:
Overall, it appears that acute exercise can induce mobilization of senescent and exhausted CD8+ T cells. The influence of repeated exercise on limiting senescent/exhausted CD8+ T cells includes: increased cardiorespiratory fitness may protect against the accumulation of senescent CD8+ T cells, endurance resistance training appears to reduce senescent CD8+ T cells in sedentary older adults, and a sedentary lifestyle may lead to an increase in exhausted CD8+ T cells. However, the number of studies available to conceive these findings was limited such that only two studies were designed appropriately to possibly generate the results in each of the three previous findings. More research is required to confirm the influence of repeated exercise on senescent and exhausted CD8+ T cells.
One explanation for this phenomenon is due to migration of T-cells to peripheral space, which may lead to their death and room for new T-cells:
Lymphocytes mobilized by acute exercise are more sensitive to apoptosis through intrinsic and extrinsic apoptotic pathways explained in detail in a review by Simpson (2011). The removal of lymphocytes, and specifically senescent T cells, purportedly creates “immune space” for the production of naïve T cells which are more immunologically responsive. It is yet to be confirmed whether senescent T cells undergo apoptosis at a greater rate than other T cell populations. Senescent T cells can be resistant to some apoptotic pathways, but evidence indicates that exercise-induced apoptotic pathways involve oxidative stress where senescent T cells may be less tolerant than other T cell populations. Even so, if senescent T cells are preferentially mobilized it is likely they are also exposed to apoptotic pathways. Frequent bouts of acute exercise and subsequent T cell shifts may have an accumulative long-term restorative effect on the immune system by “making space” for naïve T cells to increase the TCR repertoire (Simpson, 2011). Therefore, the mobilization of senescent and (some) exhausted T cells may account for the benefits of repeated exercise.
This discrepancy from prior reviews may likely be due to the available evidence at the time. The Pedersen, B. K., & Hoffman-Goetz, L. review was published around 2000 while the Donovan, et. al. review came out just last year, suggesting that this difference could be due to emerging, recent evidence that may substantiate the concept of “immune space” happening in those who exercise.
The evidence is not clear on the matter, but it does strengthen the idea that the body- through exercise- may do a little “spring cleaning” by clearing out old, ineffective T-cells while also maintaining the health and youth of current cells.
This point may be substantiated with a recent assessment from Tylutka, et. al.4 which used flow cytometry to examine the levels of CD4 and CD8 cells in those with routine exercise and those without among elderly participants, with the results suggesting that a lifestyle that includes exercise may increase levels of naïve T-cells while also maintaining an appropriate CD4/CD8 cell ratio5.
Salivary IgA and Exercise
Our immune systems create several different types of antibodies called immunoglobulins (Ig). There are 5 different types of Ig, and they can be remembered with the pneumonic device “G.A.M.E.D.”

In most discussions the two important Ig’s are IgA and IgG. IgG are the most abundant type of antibodies and circulate throughout our body to target different pathogens. IgA is another important antibody, but is found predominately within our mucosal system such as the nose, throat, and intestinal tract. It’s IgA that has been considered important in preventing respiratory infections because they are found in high levels at entryways into the body. Therefore, activation and production of IgA should infer greater protection from infection.
In many studies on exercise, most researchers measure IgA levels within saliva (salivary IgA) to measure susceptibility to infections, with an assumption that lower levels of s-IgA may be indicative of greater rates of infection.
Much of the research on s-IgA production after exercise have shown interesting results. In the elderly, routine exercise has been suggested to increase levels of s-IgA (Ntovas, et. al.6):
Older people who follow a daily moderate-intensity exercise program appear to have higher levels of S-IgA, than others of the same age who do not exercise. In addition, moderate to intense exercise can increase the secretion of salivary S-IgA in older adults to improve their immune function [52].
Similar sentiments were reiterated in a literature review from Sellami, et. al.7 suggesting that exercise among older individuals may help stimulate s-IgA production.
For younger people, the evidence hasn’t been quite clear. However, there’s been a general assumption that s-IgA production post-exercise actually declines in younger populations (Pedersen, B. K., & Hoffman-Goetz, L.):
Lower concentrations of the salivary IgA have been reported in cross-country skiers after a race (290). This finding was confirmed by a 70% decrease in salivary IgA that persisted for several hours after completion of intense, long-duration ergometer cycling (171). Decreased salivary IgA was found after intense swimming (87, 285), after running (281), and after incremental treadmill running to exhaustion (183) (Table 1). Submaximal exercise had no effect on salivary IgA (118, 183).
Such findings have led researchers to assume that the post-acute exercise period may be a time of susceptibility to infections, especially for more strenuous exercisers such as athletes:
Reduced salivary IgA concentration and secretion rate (amount of IgA secreted over a fixed period) may predispose athletes to illness in the long term (26, 35). IgA binds microorganisms such as bacteria and viruses in the mucosa so that they can be destroyed by immune cells. However, short-term changes in salivary IgA concentration and secretion rate after repeated bouts of exercise are variable (56, 58, 60, 79). Salivary IgA concentration and secretion rate may decrease incrementally over longer periods.
Such an assumption has led to the concept of an “open window” phenomenon that argues that intense exercise may be followed with a period in which people may become susceptible to infections. We’ll discuss problems with such an assumption further on.
However, as it relates to s-IgA there are many problems with these studies due to high variability among individuals and athletes, mostly because the oral environment greatly determines the concentration of IgA that is produced. This could also be influenced by the differences in exercise regimen, oral health, diet, and the environment in which the exercise is taking place (Sellami, et. al.):
A number of limitations affecting the variations of SIgA and cytokines during acute and chronic exercise should be properly acknowledged. Different modes of training interventions are obvious reasons for discrepancies, e.g., endurance training vs. resistance training; differences in the intensity of exercise; and the time duration of the single bout of exercise, as well as the training volume. In addition, a large interpersonal variability in peripheral inflammatory markers in terms of parameter like salivary flow rate, circadian rhythm, menstrual cycle, or oral health status (which sometimes are not taken into account and corrected for) (11, 140), together with a considerable coefficient of variability in high sensitivity cytokine assays make power problems common. Additional studies are needed to assess the effects of different modes and intensities of exercise on inflammation.
Because of these multitude of variables- most of which are usually not taken into account during studies- evaluation of s-IgA should be met with much skepticism. Hopefully further research may provide for consistent methodologies, but for now remember to keep this high level of variability in mind when looking at results.
Can Exercise Compromise the Immune System? The “Open Window” Hypothesis
8Although we consider exercise to be an overall net benefit to our well-being, the field of exercise immunology has been controversially plagued by the concept that exercise may be detrimental to our immune system, especially within the immediate time after a workout or routine.
Some of this evidence has been somewhat substantiated with results found in the Pedersen, B. K., & Hoffman-Goetz, L. Table, which shows that several immune biomakers may become elevated during a workout but may lessen below pre-workout levels for several hours afterwards.
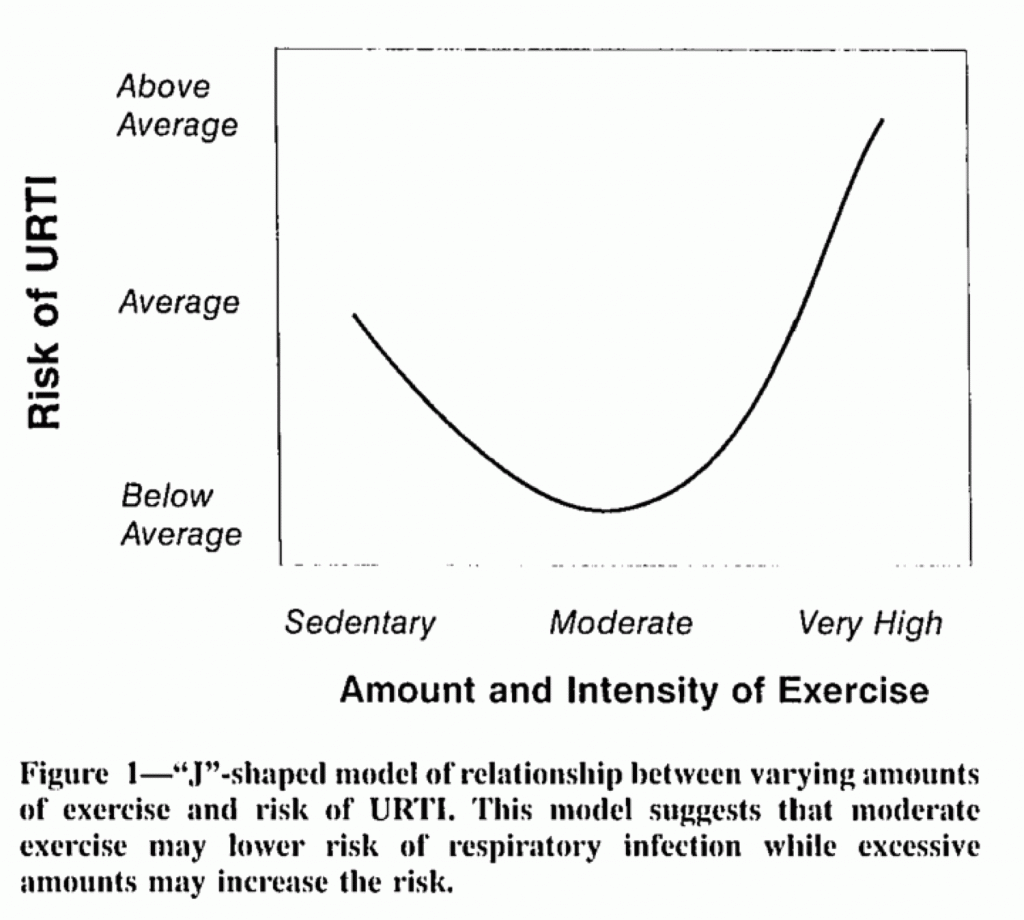
This has led many researchers to suggest that there is an immunological “open window”, such that the immune system may be depressed immediately after a workout for a short period, and within that timeframe people may become more susceptible to illness. This has led many researchers to remark that there is a “J-curve” to susceptibility.
Here’s one definition for the open window provided by Peake, et. al.:
A classic paradigm in exercise immunology is that an “open window” of immunodepression can occur during recovery from intense exercise. In particular, this paradigm proposes that after intense exercise, some immune variables (e.g., lymphocyte and natural killer cell numbers and antibody production) transiently decrease below preexercise levels. As a result of this immunodepression, microbial agents, especially viruses, may invade the host or reactivate from a latent state, leading to infection and illness (87). If exercise is repeated again while the immune system is still depressed, this could lead to a greater degree of immunodepression and potentially a longer window of opportunity for infection (87).
Some evidence suggests that immediately after a workout lymphopenia (low lymphocyte count) may be prominent, and thus may provide a window of opportunity for infection:
By contrast, lymphocyte number decreases rapidly after exercise. Following prolonged and/or high-intensity exercise in particular, lymphocyte number commonly decreases to below the preexercise value within as little as 30 min (126). This “lymphopenia” can often reach levels typical of clinical lymphopenia (<1.0 × 106/ml), but the lymphocyte count is usually restored to both the resting and clinically normal level within 4−6 h of recovery (126).
Paired with low s-IgA levels post-workout, many researchers may be led to infer that such a phenomenon as an open window may be occurring (Ntovas, et. al.):
As far as the relationship between exercise and s-IgA, the majority of the studies conclude that s-IgA decreases after exercise [27,134], others report no change [19,46] and others show increased levels of s-IgA post-exercise [17,43,50]. S-IgA measurement seems to be a good way which shows the over-training [135,136]. The s-IgA may decrease over prolonged periods of intensive training in elite athletes. This reduction is attributed to neurohormonal factors related to physical and psychological stress during intensive daily exercise [49]. In addition, no significant association between changes in s-IgA levels and those in cortisol levels during exercise [49,56]. Low temperatures, such as in ski races, might depress the activity of secretion of s-IgA [57].
High training loads can decrease s-IgA and suppress immune function, as lower concentrations of salivary IgA or chronic salivary IgA deficiencies are associated with an increased frequency of upper respiratory tract infections (URTI) [36,137]. However, more studies are required to clarify the relationship between the components of the saliva and the incidence of URTI. The coaches can use this information to predict athletes’ immune function to help reduce the risk of upper respiratory tract infection [42]. S-IgA fluctuation is seemed to be mirrored by the secretion of salivary free light chains [41].
However, we’ve gone over a few problems with these assessments already.
Migration of lymphocytes post-workout may be indicative of lymphopenia at work, however there are questions as to why this may be occurring. We’ve noted above that this migration may actually be due to maintenance of T-cells such that older, senescent T-cells may move to peripheral regions and undergo apoptosis to make room for new T-cells. There may be immunosurveillance at play that may deploy active lymphocytes, although the reason for this is not entirely clear (i.e. is it to prevent emergence of latent virus due to immunedepression, or is it due to beneficial immunological factors at work).
For s-IgA, there are many problems with how these antibodies are collected and measured, and so there may be a concern with how many results are extrapolated and interpreted.
In short, although the open window hypothesis seems to have merit based on observational data, the actual scientific evidence is not quite there to support such a claim. However, such remarks still live on, and it’s rather hard to find papers on exercise immunology (or anything in regards to exercise and the immune system) that don’t provide some space to the concept of the immunological open window.
As a rebuttal, there is a thorough review from Campbell, J. P., & Turner, J. E.9, which argues that the open window is a heavy flawed hypothesis. Although I’m taking a few excerpts from this review, I suggest people examine the review for more criticisms of the open window hypothesis.
One study that appears to have substantiated the open window theory was a study from Spence, et. al.10 in which researchers measured incidences of upper respiratory illness (URI) in elite athletes. Note that this is illness, not infection, which different in nature.
This study tracked elite athletes over several months and took nose and throat swabs from participants when they reported to have symptoms of an URI. The findings have indicated a higher incidence of upper respiratory illness among elite athletes, leading to the adoption of a so-called “J-curve” to capture this scenario.
However, there were a few problems with this study (Campbell, J. P., & Turner, J. E.):
Clarifying this issue, a study employing nasopharyngeal and throat swabs in athletes who reported URTI symptoms over a 5-month period—including periods of competition—found that few of the self-reported infections were of bacterial, viral, chlamydial, or mycoplasmal nature (20). Indeed, of 37 episodes of URTI reported by athletes in this study, only 11 of these (30%) had a positive laboratory diagnosis. These findings place the previously discussed marathon studies in a different light (17, 18) and open the possibility that many of the URTIs reported were a symptom of other non-infectious causes. Indeed, of the of the non-infectious “URTIs” reported by Spence et al. (20), and likely captured elsewhere (17, 18), it is proposed that these symptoms are a result of other causes, including allergy and asthma, non-specific mucosal inflammation, or airway epithelial cell trauma due to increased ventilation or exposure to cold air (21). In the few cases of clinically confirmed URTIs, these appear to be from viruses—in particular rhinoviruses (i.e., the “common cold”)—rather than bacterial infection (20, 22), which is in line with the typical incidence and etiology of infections at the population level (23).
For many of these studies, there’s an issue in which researchers can’t fully substantiate the claim that athletes have, in fact, become infected and have not just had an incident of a runny nose or itchy throat due to other reasons. Because of this lack of clarity, it’s hard to argue that people are more inclined to become infected after rigorous exercise. Other factors, including large social gatherings, air temperature and humidity, and genetic predispositions that may be factors in these scenarios rather than the rigorous exercise itself.
And as noted by Campbell, J. P., & Turner, J. E., there are actual reports that suggest rigorous exercise may reduce infection risk:
Contrary to the aforementioned reports that exercise heightens infection incidence, it is often overlooked that other studies indicate that exercise participation may in fact reduce the incidence of infections. For example, a recent prospective cohort study of 1,509 Swedish men and women aged 20–60 years found that higher physical activity levels were associated with a lower incidence of self-reported URTIs (35). A much smaller but very detailed analysis of illness records kept by 11 elite endurance athletes over a period of 3–16 years showed that the total number of training hours per year was inversely correlated with sickness days reported (36). Similarly, another study of swimmers monitored for 4 years found that national level athletes had higher incidence of infections than more elite international level athletes (37). Finally, studies of ultramarathon runners, who undertake the largest volume of exercise among athletes, have shown that these individuals report fewer days missed from school or work due to illness compared to the general population.
And so the evidence is not fully in favor of the open window hypothesis based on incidences of infection/illness alone.
This also appears to be the case with measurements of s-IgA, which we have covered before but have been reiterated by Campbell, J. P., & Turner, J. E., such that the evidence also doesn’t support findings of low s-IgA after exercise:
For example, in alignment with prior observations, it was found in trained runners that IgA secretion rate decreased by 25% from pre-marathon to 90 min post-marathon (55). Likewise, in a separate study, a 20% reduction in IgA secretion rate was observed in elite athletes after a 2-h rowing exercise session (56). Several other studies of similar design reported analogous findings (57–59); however, contradictory findings are also in abundance but are much less cited in the literature. Indeed, an elegant study exploring the effects of different exercise intensities, including moderate- and high-intensity exercise to exhaustion, found that although saliva flow rate decreased, IgA secretion rate actually increased in response to both of the exercise bouts. In the words of the authors, exercise to exhaustion has an “effect on the quantity of saliva, but not the quality of saliva” (60). Many other studies have also reported that exercise does not elicit a decrease in IgA secretion rates following exercise (61–66).
So what exactly is going on here to lead to the so-called consensus of the open window hypothesis among exercise immunologists? It sort of feels like what is happening here are things we have seen before in regards to OAS, such that spurious findings that may coalesce under one idea may be used as method of validating said idea.
The open window hypothesis isn’t fully substantiated by the literature, which instead either finds contradictory findings or suffers from flawed methodology. Yet the assumption lives on and told repeatedly, much to the same extent that OAS has.
This may just be a general reflection of how science based on consensus is a grossly flawed argument. Consensus is just as likely to be derived from bad science has any individual study. And so, it is not the consensus that we should refer to, but the individual studies and whether there are concerns in methodology.
More importantly, we know of all the benefits from exercise, and messaging from ideas such as the open window hypothesis can easily be used as an argument to dissuade people from exercising. Would you knowingly exercise if you thought that you may become more susceptible to getting sick? It’s already difficult to get people to exercise, and so we should be careful in pushing ideas that would cause greater hesitancy. Remember that many of these studies that “supposedly” suggest the open window is occurring are occurring in top athletes, and not people who are trying to live an average, active lifestyle. So even if the open window is a concern, it’s hardly likely to be a concern for a large portion of the population, and it certainly shouldn’t be used as an excuse to not exercise in the first place.
So here we dove a bit into the field of exercise immunology. There’s clear associations between exercise and immune health, and here we highlighted a few points (although not exhaustively).
The field has been around for many years, but it suffers from many issues that other exercise science fields suffer from. Low sample sizes, inconsistent methodologies, and issues with interpretation and extrapolation of results may cause scientists to incorrectly seek out wrong hypotheses.
Regardless, the evidence here should provide evidence as to the benefits of exercise on our immune system. It should remind us that an active lifestyle is part of a healthy lifestyle, and we should encourage ourselves to engage in more exercise and activities. If there are concerns over the open window, remember that moderation is key and to not overwork yourself. Much of the evidence suggests over exertion may result in a net negative effect.
Overall, exercise in one way in which we can better ourselves and our health, and at a time of great uncertainty we should make sure to become more active.
The next post will provide a quick rundown of the benefits of exercise with respect to COVID.
If you enjoyed this post and other works please consider supporting me through a paid Substack subscription or through my Ko-fi. Any bit helps, and it encourages independent creators and journalists outside the mainstream.

Pedersen, B. K., & Hoffman-Goetz, L. (2000). Exercise and the immune system: regulation, integration, and adaptation. Physiological reviews, 80(3), 1055–1081. https://doi.org/10.1152/physrev.2000.80.3.1055
Peake, J. M., Neubauer, O., Walsh, N. P., & Simpson, R. J. (2017). Recovery of the immune system after exercise. Journal of applied physiology (Bethesda, Md. : 1985), 122(5), 1077–1087. https://doi.org/10.1152/japplphysiol.00622.2016
Donovan, T., Bain, A. L., Tu, W., Pyne, D. B., & Rao, S. (2021). Influence of Exercise on Exhausted and Senescent T Cells: A Systematic Review. Frontiers in physiology, 12, 668327. https://doi.org/10.3389/fphys.2021.668327
Tylutka, A., Morawin, B., Gramacki, A., & Zembron-Lacny, A. (2021). Lifestyle exercise attenuates immunosenescence; flow cytometry analysis. BMC geriatrics, 21(1), 200. https://doi.org/10.1186/s12877-021-02128-7
Generally, healthy individuals should have a higher level of CD4+ cells compared to CD8+ cells. CD4+ cells generally deal with an active infection while CD8+ cells may deal with cancer growth. The ratio between the two can tell whether something may be going wrong with the body. A low CD4/CD8 ratio, usually below 1 can indicate severe immune dysfunction such as those caused by an HIV infection or immunosenescence where CD4+ cells may become depleted. However, a very high CD4/CD8 ratio may indicate an active infection. Generally, a ratio between 1-4 is considered a normal ration.
Ntovas, P., Loumprinis, N., Maniatakos, P., Margaritidi, L., & Rahiotis, C. (2022). The Effects of Physical Exercise on Saliva Composition: A Comprehensive Review. Dentistry journal, 10(1), 7. https://doi.org/10.3390/dj10010007
Sellami, M., Bragazzi, N. L., Aboghaba, B., & Elrayess, M. A. (2021). The Impact of Acute and Chronic Exercise on Immunoglobulins and Cytokines in Elderly: Insights From a Critical Review of the Literature. Frontiers in immunology, 12, 631873. https://doi.org/10.3389/fimmu.2021.631873
Scudiero, O., Lombardo, B., Brancaccio, M., Mennitti, C., Cesaro, A., Fimiani, F., Gentile, L., Moscarella, E., Amodio, F., Ranieri, A., Gragnano, F., Laneri, S., Mazzaccara, C., Di Micco, P., Caiazza, M., D'Alicandro, G., Limongelli, G., Calabrò, P., Pero, R., & Frisso, G. (2021). Exercise, Immune System, Nutrition, Respiratory and Cardiovascular Diseases during COVID-19: A Complex Combination. International journal of environmental research and public health, 18(3), 904. https://doi.org/10.3390/ijerph18030904
Campbell, J. P., & Turner, J. E. (2018). Debunking the Myth of Exercise-Induced Immune Suppression: Redefining the Impact of Exercise on Immunological Health Across the Lifespan. Frontiers in immunology, 9, 648. https://doi.org/10.3389/fimmu.2018.00648
SPENCE, LUKE1; BROWN, WENDY J.1; PYNE, DAVID B.2; NISSEN, MICHAEL D.3-6; SLOOTS, THEO P.3-6; MCCORMACK, JOSEPH G.7; LOCKE, A. SIMON1,8,9; FRICKER, PETER A.2. Incidence, Etiology, and Symptomatology of Upper Respiratory Illness in Elite Athletes. Medicine & Science in Sports & Exercise: April 2007 - Volume 39 - Issue 4 - p 577-586
doi: 10.1249/mss.0b013e31802e851a



Great article. It occurs to me that after "exercise" I feel better, positive, ready to do more. The release of endocanabinoids, changes in cortisol, and endocrine hormones, plus all the changes that no one knows about, have the effect of placebo (jmho) against depression, pain, stress, etc. Like I said in the last post, do it for it's fun aspect, and feels good, rather than having to work out
This looks like an awesome and theory-heavy review! I will be sure to read it at my weekly beach break tomorrow.
Skimming it, I already read the part about IgA depletion. By coincidence I had just finished a 70 minute swimming session (non-continuous) so I have spent the rest of this evening in hypochondriac mode, obsessing over whether my throat feels scratchy, haha.
I have theories on this subject, naturally. Even though wouldn’t say it’s necessary to explain immune suppression from an evolutionary lens - it could just be a bug in the hardware - it’s definitely possible that it confers fitness to “lower the [innate] defenses” when not otherwise ill/weak (which usually precludes exertion) in order to court infection and generation of adaptive immunity. It is the host’s way of “feigning vulnerability” to microbes.