A quick look at Zinc
As colder weather approaches, let's take a look at some supplements that may help improve our health.
So apparently in my neck of the woods it’s beginning to feel a lot like Christmas, and not in a good way.1
In recent weeks the temperature has dropped significantly, possibly up to 20-30 degrees compared to the 70s that we experienced mid-late September.
It’s possibly one of the coldest Octobers that I can recall, although I will preface this by saying I don’t have many Octobers under my belt to use as a comparison.
Anyways, let’s just say that I wasn’t prepared for the sudden drop in temperature and the constant rainy days which have started to make me feel slightly under the weather.
Considering that many people may be facing a much colder October as well, I thought it would be a good time to revisit some supplements that may be help with the coming cold weather and concerns over getting sick, including the concerns over COVID infection spikes or variants.
In this case I wanted to take a look at little old, cationic Zinc. I never quite understood the antimicrobial nature of an ion, however given that it’s been argued that Zinc is a big fighter in pathogens then it may be worth taking a look at what exactly is responsible for this antimicrobial mechanisms.
A Zinc Overview
Zinc is considered to be one of the most vital trace elements for our overall health, coming in second in abundance behind iron.
Its effects on the body are multifaceted, being involved with bone development, cell proliferation and division, works as a chelator and stabilizer of enzymes, cell signaling, and immune function.
Low levels of zinc has been associated with many maladies such as cancer, diabetes, and infectious diseases such as HIV and the flu.
Zinc exists in the plasma at low levels due to the chelation of zinc with serum proteins. However, the exchange between chelated zinc and free zinc can happen rapidly.
Although the body does not have large zinc reservoirs and must require daily intake of zinc, many proteins may serve as chelating agents that help for short-term storage of zinc such as metallothionein and albumin.
Zinc Sources
We tend to get most of our zinc from animal sources such as meat, eggs, dairy, and seafood. Several plant sources such as beans, nuts, wheat, and rice also contain zinc. However, these sources also are rich in phytates, which are compounds that act as chelating agents for cations such as zinc and may reduce the bioavailability of zinc from these foods.
In fact, it’s argued that phytates may be associated with zinc deficiency, and that the consumption of foods rich in phytates such as breakfast cereals may contribute to a deficiency in zinc due to the phytate levels. Interestingly, many breakfast cereals may be fortified with zinc, so the overall issue with phytate consumption is a bit nuanced. We’ll explore this topic in a bit more detail further down.
Daily Intake Allowance and Symptoms of Excessive Zinc
The Recommended Dietary Allowances (RDAs), as reported by the NIH, suggest the following daily intake of zinc given age and pregnancy/lactation status. Remember that this is a general guideline and likely to vary from person to person.
As with anything, excessive intake of zinc may lead to unwanted symptoms, including nausea, upset GI, headaches, dizziness, and vomiting. Some of this may come from excessive supplementation, although apparently denture-adhesive creams may also be a source of high zinc as well. High levels of zinc has also been associated with reduced copper which may cause anemia and neurological impairment.
The NIH suggests these upper-limits for zinc daily intake, but once again remember that these numbers are likely to vary between individuals.
It’s important to be on the lookout for physiological changes when supplementing, and it’s worth consulting a medical professional for additional advice.
Groups at-risk for Zinc Deficiency
Zinc bioavailability is influenced by age, sex, and pregnancy status. In general, older populations are more likely to be at risk for zinc deficiency, possibly due to reduced absorption of nutrients and reduced GI function, or due to comorbidities that may affect zinc levels.
A few more groups include the following, although note that there are likely to be more at-risk groups that aren’t listed below:
People with chronic GI issues: People with inflammation of the intestines or impaired GI function may have reduced intestinal absorption of zinc. Zinc supplementation may help these individuals, although consultation with a medical professional would be advised.
Vegetarians/Vegans: Because zinc is sourced predominately from meat and animal products vegetarians, especially vegans, may not obtain enough zinc from their diet. This issue may be exacerbated by the fact that most zinc-rich plants such as legumes and wheat may contain high levels of phytates and may reduce the bioavailability of zinc. Those eating an exclusively plant-based diet should keep in mind the foods they eat and the possible consumption of high levels of phytates in contrast to their zinc intake (we will discuss phytates a bit more down below and whether these concerns are actually warranted).
Pregnant/Lactating Women: Pregnancy may increase the demands for nutrients which are sourced to produce breastmilk and feed infants, or to help a fetus grow and develop while in utero. As such, it is recommended that pregnant and lactating women increase their daily intake of zinc in order to compensate (the Food and Nutrition Board, or FNB recommends an increase of 3 mg/day for pregnant women and 4 mg/day for lactating women). Remember that it’s important to consult with medical professionals to see what changes in daily intake would be right for you.
Alcohol consumption: Excessive consumption of ethanol may impair intestinal absorption of zinc while also elevating zinc excretion through urine.
Are Phytates a real concern?
Phytates, also called phytic acid are complexes derived from inositol and comprised of 6 phosphate groups branching off of the central ring. Plants such as legumes, rice, and wheat use phytates as a way of storing phosphates for later use. As a ring of negatively charged phosphates phytate can interact with cations such as magnesium, iron, calcium, and zinc.
An example of this zinc phytate complex can be seen below:

Phytates are indigestible, meaning that their structure can’t be broken down through regular digestion. This can create an issue, in that phytates may chelate zinc from other food sources and prevent them from being properly absorbed by the body.
It also would mean that phytates which may chelate plant cations may not release these cations to us, meaning that zinc from plants containing phytate complexes would be unavailable for us.
This phenomenon has given certain compounds the name of “anti-nutrient” since it’s assumed that these compounds may actually extract vital nutrients from those who ingest them. This designation, as noted in a review by Petroski, W., & Minich, D. M.2, would suggest that certain compounds in isolation may end up being detrimental on the body rather than beneficial.
Luckily, as noted in the review, phytates are readily broken down through fermentation, cooking, and even through plant enzymes such as phytases that cleave the phosphate groups off of phytate and thus increases the bioavailability of plant cations such as zinc.
Given the fact that phytates are likely not to be eaten alone, that most sources are likely to be processed in some manner, and that phytates may exert their own antioxidant properties it’s unlikely that phytates would prove detrimental unless one eats a raw diet consisting primarily of phytate-rich foods.
It doesn’t necessarily mean that phytates may be a concern, but it’s important to take a look at the foods you eat and understand the totality of your diet when considering whether phytates are the sole cause of zinc deficiency.
Zinc Mechanisms of Action
We’ll focus on two important mechanisms of zinc: zinc’s direct action on pathogens and zinc’s role in immune function.
Note that these remarks are broad and won’t encompass some of the finite details when it comes to zinc, so use this as an general introduction which may be elaborated on in later articles.
Zinc as an antimicrobial
Zinc has several mechanisms to target pathogens, such as by targeting proteins necessary for membrane fusion or by targeting enzymes critical for viral function.
Although there are differences in how zinc may target viruses, bacteria, and parasites the focus here will examine viruses, albeit in a general manner.
In vitro studies has elucidated several of these antiviral mechanisms of zinc, with one overview from Read, et al.3 summarizing some of the findings from various studies looking at the antiviral nature of zinc. Some of these actions include the targeting of viral proteases and polymerases, while also inhibiting viruses from endosomal membrane fusion.

One critical problem with in vitro studies is that many studies tend to use concentrations above the physiological concentrations, meaning that the effects may not be translatable to real-world settings.
Read, et al. notes some of these challenges in this way:
Unfortunately, zinc concentrations used to assess antiviral activity often far exceed physiological concentrations. Human plasma zinc, for example, ranges from approximately 10 to 18 μM (47), whereas antiviral concentrations of zinc can reach into mM concentrations (48). Intracellular zinc concentrations range from 10s to 100s of μM, but are significantly buffered by zinc-binding proteins such as metallothioneins, rendering free zinc concentrations at picomolar to low nanomolar concentrations (49, 50).
This is a general problem of in vitro studies which may not translate to real-world settings.
However, Read, et al. also includes this chart on antiviral clinical trials and zinc supplementation:

Although the clinical trials here only show evidence for common colds as an indicator for respiratory infections, it’s important to note that zinc in these trials was administered as a lozenge.
Clinical trials may utilize lozenges and nasal sprays that may coat mucosal membranes within the nose and throat. Zinc administered in this manner may prevent virions from properly attaching to the receptors of cells.
At this time I haven’t found the proper explanation for this mechanism of action. It could be that abundance of zinc may lead to exchange with other chelating ions that form salt bridges or other complexes on the surface of viral proteins and alter critical binding interactions, a phenomenon called mismetallation. Zinc may also chelate to various regions and cause conformational changes to viral proteins that prevent proper binding of the virus to host receptors.
Either way, many studies on respiratory infection appear to utilize throat lozenges or nasal sprays, suggesting that the route of administration of zinc during times of infection may be critical in attenuating infection, although it’s worth noting that the actual reduction time of symptoms in trials that utilize throat lozenges for the common cold vary widely from either improving days of infection or possibly extending it.
Such action may be attributed to the form of zinc used which may show differences in bioavailability and may be affected by pH.4
Nutritional Immunity and the Complexities
However, this brings up a rather complex scenario, in that zinc is generally spoken of as an antimicrobial when in reality many pathogens may steal zinc and other ions from the host’s body in order to utilize it for its own replication and survival.
For instance, several viruses such as human papilloma viruses may actually alter zinc homeostasis and use such mechanisms in order to increase viral gene expression.
It’s not known to what extent other viruses can alter homeostatic levels of zinc, however it’s known that bacteria undergo competition with the host to battle for necessary nutrients such as trace minerals.
Since many bacteria require zinc for their cellular functions they may attempt to syphon available zinc from their host.
Apparently humans have developed a way of sequestering minerals to reduce their bioavailability for microbes. This mechanism, called nutritional immunity, outlines various mechanisms in which the body may shuttle ions such as zinc, magnesium, and iron (which was the initial ion first examined for this phenomenon) away from circulation and into proteins and cells so that pathogens may not be able to use these ions for themselves.
This mechanism also appears to be a key factor in cytokine release and pro-inflammatory responses that occur during the acute stages of an infection, indicating a dynamic interaction between inflammation and the mobilization of ions.
One schematic is provided by Lonergan, Z. R., & Skaar, E. P.5 that shows this mobilization of zinc during an acute infection. It's important to note that this phenomenon outlined below is based on the acute phase of a bacterial infection, and may not be indicative of a phenomenon that occurs during viral infections due to the need for viruses to hijack and utilize host cell machinery:
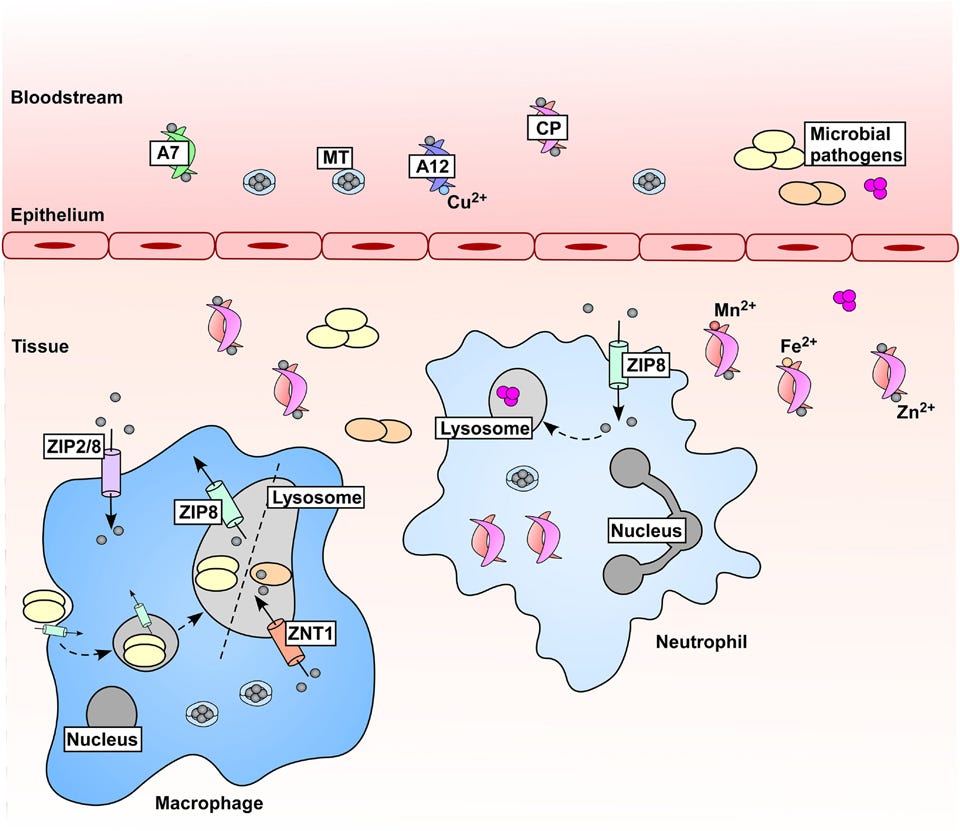
This creates a rather complex dynamic. This suggests that the innate immune system may try to remove ions at the same time pathogens may try to use them.
It creates a rather paradoxical phenomenon: could supplementation of zinc during an infection help improve and alleviate symptoms, or could it exacerbate them?
In vitro assays may not be able to properly capture the mechanisms of nutritional immunity, and clinical trials may indicate other dynamics at play given the fact that these clinical trials may not use oral supplements, especially for respiratory infections.
This would raise questions as to whether intracellular zinc, and the use of zinc ionophores may be viable in targeting viral infections.
As noted above, this phenomenon of deprivation via nutritional immunity may only be a phenomenon of bacterial infections since viral infections require the infection and utilization of host cells.
This distinction does allow one mechanism to be beneficial, such that the uptake of zinc by specific immune cells may actually create a toxic environment for viruses who infect such cells.
Given the fact that bacteria appear to compete for trace elements, and that this effect does not appear to happen in large degree for viruses, it could be that mismetallation of viral enzymes in a high zinc environment cannot be countered by viruses and may actually disrupt cellular functions due to inhibition of viral polymerases and proteases.
Therefore, it could be that zinc depletion via shuttling into cells may increase intracellular levels and create an antiviral environment.
All of this shows the complex dynamics that can even result from something as small as an ion.
Zinc and Immune function
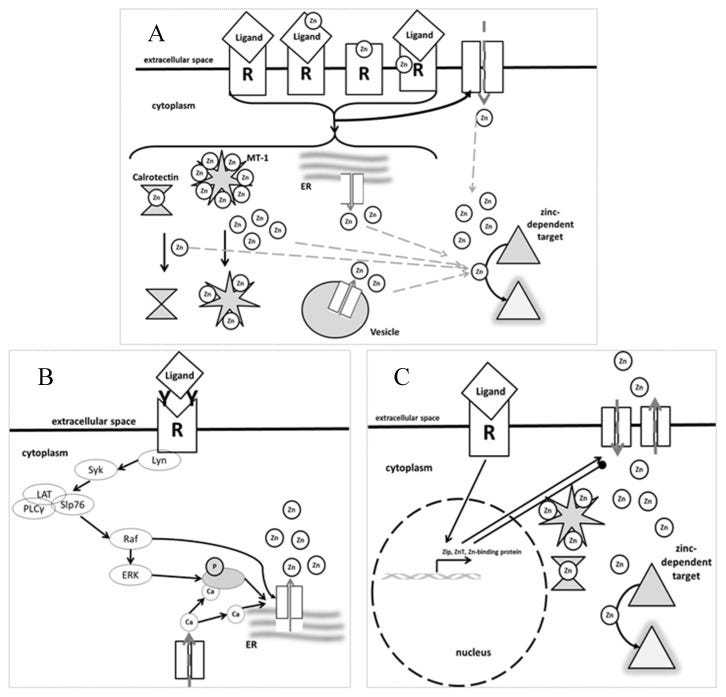
The direct antimicrobial effects of zinc is rather nuanced and relates to the pathogen.
When it comes to immune function recent research has suggested that zinc is vital to proper immune signaling pathways.
For instance, adequate zinc levels are necessary for proper innate immune responses, intracellular signaling pathways, as well as the expression of various receptors on the surface of immune cells such as T-cells.
Many of these discussions will be beyond the scope of this post, but Wessels, et al.6 provides this comment in the conclusion of their review:
Zinc flux, zinc wave, and homeostatic zinc signals control the adequate function of innate as well as adaptive immunity. On the one hand, zinc deficiency causes severe impairment of immune function, comprising the adaptive as well as the innate immune system. On the other hand, high zinc excess provokes an impairment of the immune system comparable to zinc deficiency (see Figure 5). This is why a balanced zinc homeostasis is crucial for either defending against invading pathogens or protecting the human body against an overreactive immune system causing autoimmune diseases, chronic inflammation or allergies. In this regard, zinc can be considered as a gatekeeper of the immune system, since the adequate function of virtually all immune cells is highly zinc-dependent. Knowledge about the molecular mechanisms in zinc-regulated immune reactions is growing and inherited disorders such as Acrodermatitis Enteropathica, as well as nutrition-related immunological malfunctions observed in the elderly, can be treated by appropriate zinc administration. Thus, zinc can be seen as potential therapeutic for clinical use to influence beneficially the well-being of patient’s suffering from immune diseases. However, to completely understand the complex-regulated zinc-triggered immunoreactions, more research will be necessary.
More thorough examination of these immune functions can be seen in the Wessels, et al. review as well as this review from Maywald, et al.7 Both reviews highlight the critical role that zinc plays in toll-like receptor 4 (TLR4) signaling, a critical transmembrane protein that is vital for innate immune signaling, as well as the expression of various receptors critical for immune functioning.
Altogether, there’s no doubt that zinc is necessary for proper immune function. Although the mechanisms are rather complex, the evidence appears to suggest that zinc is critical for many aspects of immune cell signaling. Given the fact that a strong immune system is vital for prevention and fighting off infections, these findings generally support the idea of making sure zinc levels are adequate prior to exposure to a pathogen.
Zinc during the coming cold spell
As we enter into the colder season and paranoia over infection begins to run abound again it’s important to find ways of protecting ourselves and aid our immune system.
Zinc is a critical trace element responsible for many physiological processes. It’s the second most abundant transitional element in the body.
It’s immunological mechanisms are complex, and this review is in no ways complete. However, the evidence does appear to suggest that proper zinc levels are needed in order to aid in immune signaling and proper cellular functions.
As a direct antiviral/antimicrobial, the situation of nutritional immunity raises questions as to what and where the zinc ends up. Proper zinc levels may provide immune cells the necessary cytotoxic effects to target viruses. However, respiratory infections may need more localized targeting of the virus, and use of nasal sprays and lozenges that contain zinc may inhibit viral proteins from attaching to cells properly.
More, extensive research would be needed to examine these factors more deeply.
However, as winter begins to arrive (in October…) we should think about ways we can help improve our health.
If you enjoyed this post and other works please consider supporting me through a paid Substack subscription or through my Ko-fi. Any bit helps, and it encourages independent creators and journalists outside the mainstream.

This is besides the fact that retailers are putting up Christmas decorations, as if Halloween and Thanksgiving are no longer holidays worth celebrating!
Petroski, W., & Minich, D. M. (2020). Is There Such a Thing as "Anti-Nutrients"? A Narrative Review of Perceived Problematic Plant Compounds. Nutrients, 12(10), 2929. https://doi.org/10.3390/nu12102929
Read, S. A., Obeid, S., Ahlenstiel, C., & Ahlenstiel, G. (2019). The Role of Zinc in Antiviral Immunity. Advances in nutrition (Bethesda, Md.), 10(4), 696–710. https://doi.org/10.1093/advances/nmz013
Eby G. A., 3rd (2010). Zinc lozenges as cure for the common cold--a review and hypothesis. Medical hypotheses, 74(3), 482–492. https://doi.org/10.1016/j.mehy.2009.10.017
Lonergan, Z. R., & Skaar, E. P. (2019). Nutrient Zinc at the Host-Pathogen Interface. Trends in biochemical sciences, 44(12), 1041–1056. https://doi.org/10.1016/j.tibs.2019.06.010
Wessels, I., Maywald, M., & Rink, L. (2017). Zinc as a Gatekeeper of Immune Function. Nutrients, 9(12), 1286. https://doi.org/10.3390/nu9121286
Maywald, M., Wessels, I., & Rink, L. (2017). Zinc Signals and Immunity. International journal of molecular sciences, 18(10), 2222. https://doi.org/10.3390/ijms18102222




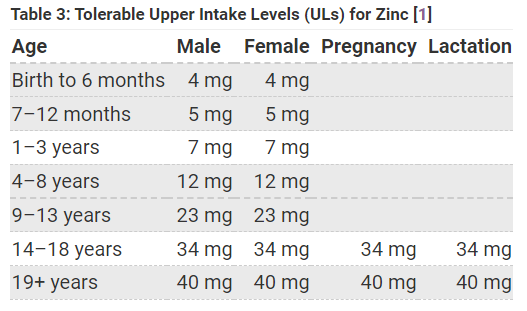
With respect to: "As with anything, excessive intake of zinc may lead to unwanted symptoms, including nausea, upset GI, headaches, dizziness, and vomiting. Some of this may come from excessive supplementation, although apparently denture-adhesive creams may also be a source of high zinc as well. High levels of zinc has also been associated with reduced copper which may cause anemia and neurological impairment."
I have been advised that it is a good idea to take 1mg of copper for every 15mg of zinc. I found this in a product called "zinc balance". Any additional thoughts on this are welcome.
Very focused piece. Hit many points. I have seen the zinc to copper ratio being important and there's only one mention of it which I found curious.