A genetic basis for asymptomatic COVID?
We've heard of many people having asymptomatic COVID. Now, do we have evidence for why this may happen?
In the early days of COVID there have been quite a great deal of controversy surrounding the concept of “asymptomatic COVID”, as in an individual testing positive for COVID and yet presenting with no symptoms.
This has raised a lot of serious questions over how such a feat can occur, with some alluding to false positives from PCR tests as being one of many possible explanations.
Now, in recent months there seems to be some evidence suggesting a genetic basis for the phenomenon of asymptomatic COVID.
HLA Overview
Yesterday my friend showed me a preprint, which now appears to have been published in Nature1:
I have mentioned HLA before in prior articles. In short, HLA serves as the line from antigen exposure to adaptive immune response by way of antigen presentation. It’s how our body comes to recognize foreign agents that we come across such as when we get infected.
You may have heard of HLA in school, only there you may have been told of the Major Histocompatibility Complex, or MHC. MHC is a broader term for this system of antigen presentation, with HLA being the human form (HLA standing for Human Leukocyte Antigen2).
The discussion on HLA can become rather complex, but to keep it simple HLA is one of the most polymorphic regions of our genomes. That is to say, HLA molecules are some of the most diverse molecules know, with thousands of different alleles being recognized3.
For those interested, an easily assessible reading on HLA can be found in a review from Rock, et al.4 A small excerpt explaining HLA includes the following:
T cells help eliminate pathogens present in infected cells and also help B cells make better and different kinds of antibodies to protect against extracellular microbes and toxic molecules. To accomplish these important functions, T cells have to interact intimately with other cells and then find and instruct or eliminate the ones that are harbouring or have been exposed to these pathogenic threats. However, T cells are unable to peek beneath the surface of cells to identify ones that have ingested bacteria or are synthesizing viral or mutant proteins. Instead, antigen presentation systems have evolved, that display on the cell surface information about the various antigens that cells are synthesizing or have ingested. These antigen presentation pathways monitor the major subcellular compartments wherein pathogens could be lurking and report their findings to the appropriate kinds of T cells. Endogenously synthesized antigens in the cytosol of all cells are presented to CD8+ T cells as peptides bound to MHC I molecules, thereby allowing the CD8+ lymphocytes to identify and eliminate virally infected cells or cancers.
HLA molecules are categorized into different classes: MHC Class I and MHC Class II.
Class I molecules can be further subdivided into alleles such as A, B, and C. This class is found across most cells in our bodies, and they are pivotal in presenting intracellular proteins to immune cells. Again, this process is a bit too complex for this discussion, but what essentially occurs is that proteins produced within a cell are chopped up by proteasomes. The resulting peptides are then carried out to the surface of the cell and presented by Class I alleles.
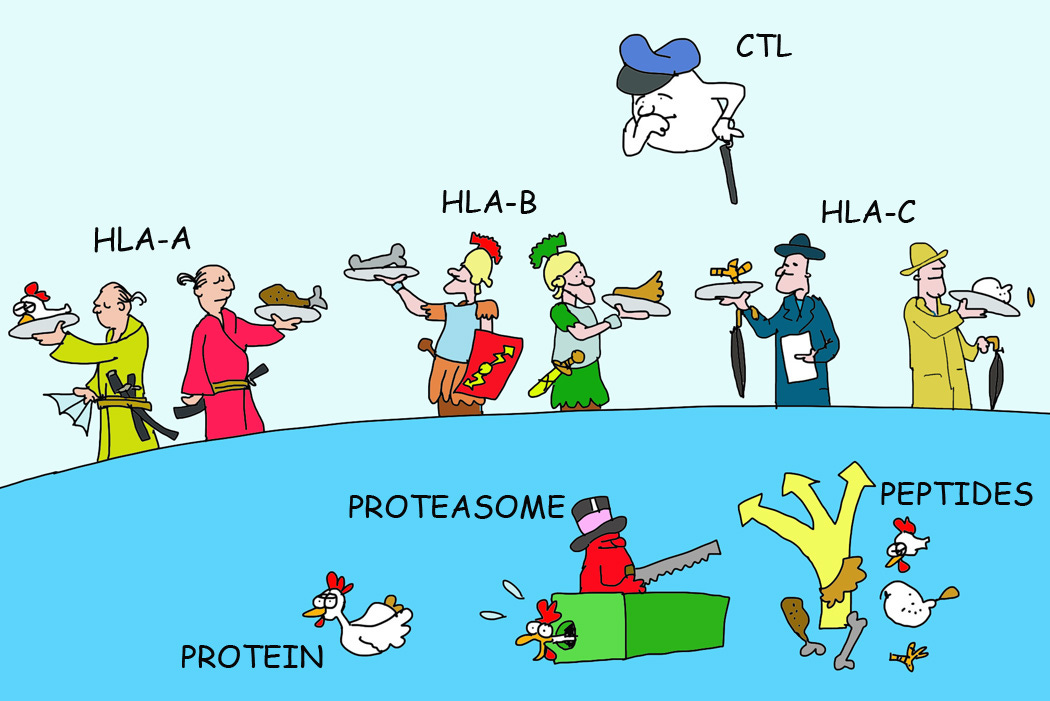
If these proteins are autologous (that is, normally produced by the body), then immune cells are made to be tolerant of these peptides and disregard their presentation. However, if these peptides don’t seem normal then the immune system is told to respond and act. In this case, the cells presenting these off-peptides are then killed off by cytotoxic T-cells. There’s a constant ebb and flow of antigen presentation and recognition by the immune system, a constant battle of “check yourself before you wreck yourself”, so to speak.
Class II molecules are a bit more specific, being found predominately on immune cells and epithelial cells. In this case, these molecules are not intended to lead to destruction of cells, but rather the activation of immune cells. Class II molecules present antigens to naïve T cells and B cells, which cause them to activate and mount a response to these peptide-carrying agents. In that regard, Class II molecules act almost as wanted posters, presenting the body with images (peptides) that the immune system should look out for.
For now, the discussion on Class II molecules is not necessary, so I will once again refer readers to Rock, et al. for an overview with more cheeky illustrations.
All things considered, HLA molecules are pivotal for immune function, with different alleles being associated with various diseases, including autoimmune diseases such as celiac disease.
In the case of COVID, it’s been argued that people carrying the HLA-B*46:01 allele may be more susceptible to severe infection. The reasoning is rather interesting, as it’s been suggested that this allele in particular may not recognize peptides from SARS-COV2. Again, the immune system cannot target what it cannot see, and so if this allele cannot present any peptides then the immune system won’t know that anything is really off as cells continue to get infected.
In contrast, the study presented by Augusto, et al. argues that one allele, HLA-B*15:01, may be protective against COVID by way of cross-reactivity, in that this allele may recognize an epitope found in seasonal coronaviruses that are also similar to ones found in SARS-COV2. Thus, prior exposure to seasonal coronaviruses may serve as a priming event, with exposure to SARS-COV2 being the quick memory recall event that results in lack of symptoms.
Study Overview
The study relied on volunteers with genotyping for HLA alleles found in the National Marrow Donor Program (NMDP). Volunteers were asked to fill out surveys providing baseline characteristics, which included questions on whether a patient tested positive for SARS-COV2. Note that this study took place in early 2020 up until April 2021- prior to any vaccine rollout.
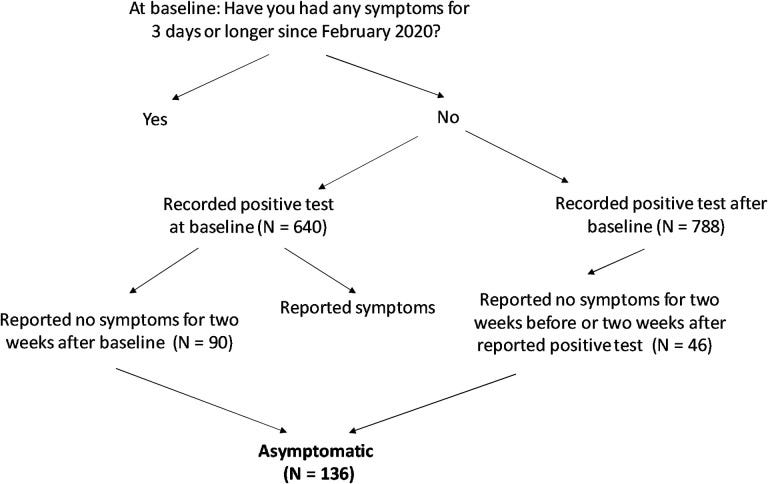
Patients who responded no to all questions on symptoms, who also self-reported a positive test were included as being “asymptomatic”. An outline from the pre-print of this study shows how patients were included:
Note that SARS-COV2 positivity and symptoms were all self-reported, and so there’s likely to be a high degree of subjectivity with these results. Note that the study was narrowed to include anyone who self-reported as being “white” due to limited demographic data. Different ethnicities are known to carry different HLA alleles, and so the narrowing of participants to one demographic means that these results may only be representative of people who have European ancestry and won’t tell us much about alleles prevalent in other racial groups that may be protective. Also, note that the sample size is relatively small. Given the thousands of different combinations of alleles possible it’s very likely that this small sample size is not representative of all known B loci combinations, as well as other loci.
Now, when comparing allele frequency among asymptomatic volunteers compared to symptomatic volunteers the researchers noted a high association with people carrying the HLA-B*15:01 allele, which appears to be stronger in those carrying two copies of these alleles. However, note that the percentage difference between the two groups is rather small. Only 20% of asymptomatic volunteers carried this allele compared to 9% of volunteers who were reported as symptomatic. However, assessment of two separate cohorts also appeared to note a higher prevalence of this allele among asymptomatic volunteers.
So far, this would only appear to be a correlative finding, which may not tell us much about actual cross-reactivity.
In order to find more specific evidence, the researchers used 4 different epitopes5 from SARS-COV2 that have been found to be bound by HLA-B*15:01 (CVADYSVLY, HVGEIPVAY, NQKLIANQF and RVAGDSGFAAY) and combined them with pre-pandemic peripheral blood mononuclear cell (PBMC) samples from 9 patients carrying this allele. The intent here was to see if T-cells from people who have never been exposed to SARS-COV2 will show some degree of reactivity.
This does appear to be the case, with two SARS-COV2 derived epitopes (CVADYSVLY and NQKLIANQF) in particular inducing a memory T-cell response.
Augustus, et al. notes the following in particular with the epitope NQKLIANQF (emphasis mine):
NQKLIANQF (hereafter, NQK-Q8) was detectable in the highest proportion of samples (5 out of 9; 55.6%). Notably, in those donors, 100% of ex vivo NQK-Q8 tetramer+CD8+ T cells were memory T cells, indicating pre-existing T cell immunity against SARS-CoV-2 in a subset of individuals carrying HLA-B*15:01 who did not have any previous contact with the virus (Fig. 1c and Supplementary Table 5).

Note again that we are referring to memory here. In this case, memory suggests that some of the 9 patients had to have been exposed to something with this similar epitope in the past in order elicit this response.
So what exactly could have been the prior exposure agent? It appears that the one epitope in particular noted above (NQK-Q86) is quite homologous to epitopes from seasonal coronaviruses, most notably HKU1-CoV and OC43-CoV.
This suggests that people who may have been previously exposed to seasonal coronaviruses who also bear this HLA allele may be quick to respond to SARS-COV2, leading to quicker viral clearance and reduced symptom onset by way of cross-reactivity and prior memory of this NQK epitope.
Note that the only difference between these two epitopes appear to be the amino acid in the 8th position, with the SARS-COV2 derived epitope bearing a glutamine (Q) while the two seasonal coronaviruses listed above bearing an alanine (A).

There are slight variations in side-chain structure between these two amino acids. Alanine is a simple methyl group, whereas glutamine bears an amide functional group.7
Nonetheless, these differences don’t appear to alter the binding of HLA-B*15:01 to these alleles based upon a thermal melt assay. A thermal melt assay notes the heat needed in order to stop two proteins from annealing to one another. This is reflected in the fluorescence measured, whereby higher fluorescence suggests higher degree of HLA antigen/epitope annealing. Past that point, the temperature becomes high enough that the proteins can become separated and fluorescence is lost.
This is illustrated in Figure 4a, which notes that both epitopes have similar melting points, suggesting that the swap between a glutamine and an alanine residue don’t seem to contribute much to the binding of this epitope to the corresponding HLA allele.

There’s more to immunity than antibodies
I’ll leave the rest of the study for readers to examine if interested. The study goes further to assess cross-reactivity and provides more evidence.
However, note that there has been some speculating that prior exposure to coronaviruses may confer cross-reactive immunity against SARS-COV2. This study seems to provide a genetic basis for this possibility, whereby those who carry HLA alleles that recognize epitopes derived from both SARS-COV2 as well as seasonal coronaviruses can exhibit cross-reactive immunity. This tells us, again, that there is far more to immunity than just antibodies, and that the continues need to check for antibody titers is not a fair assessment for what actually goes on in the body.
More importantly, this study raises a critical question of not just natural immunity from SARS-COV2, but whether prior immunity to other viruses may contribute to protection and less severe illness in the wake of the pandemic.
Note that there’s a few issues with this study. The volunteers don’t seem to be tested for prior coronavirus exposure, let alone actual SARS-COV2 exposure aside from self-surveys. The association with asymptomatic individuals appear to be more temporally associated given that carriers of this allele appear in both symptomatic and asymptomatic groups, which may be due to subjectivity of responses. Further studies with more objective data collection can provide more insights.
Personally, I’m not too convinced about this association with asymptomatic infection, although I would argue that it at least provides a better explanation that ones that we have been given beforehand. It also serves as a reminder that more should be looked at than just antibodies. After all, the immune system is far more complex than we give it credit for.
Substack is my main source of income and all support helps to support me in my daily life. If you enjoyed this post and other works please consider supporting me through a paid Substack subscription or through my Ko-fi. Any bit helps, and it encourages independent creators and journalists such as myself to provide work outside of the mainstream narrative.

Augusto, D.G., Murdolo, L.D., Chatzileontiadou, D.S.M. et al. A common allele of HLA is associated with asymptomatic SARS-CoV-2 infection. Nature (2023). https://doi.org/10.1038/s41586-023-06331-x
Note that some of the figures were taken from the preprint, which don’t appear in the full article. Anything used from the preprint in this post will be noted as being from the preprint.
For those interested the preprint can be found below:
Augusto, D. G., Yusufali, T., Sabatino, J. J., Jr, Peyser, N. D., Murdolo, L. D., Butcher, X., Murray, V., Pae, V., Sarvadhavabhatla, S., Beltran, F., Gill, G., Lynch, K., Yun, C., Maguire, C., Peluso, M. J., Hoh, R., Henrich, T. J., Deeks, S. G., Davidson, M., Lu, S., … Hollenbach, J. A. (2022). A common allele of HLA mediates asymptomatic SARS-CoV-2 infection. medRxiv : the preprint server for health sciences, 2021.05.13.21257065. https://doi.org/10.1101/2021.05.13.21257065
To reiterate this point, remember that the word antigen does not refer to foreign agents only. Antigen is a portmanteau of “antibody generator”, and so it refers to anything that induces an immune response, including anything derived from our own bodies.
Remember that HLA molecules are inherited by both parents since it resides on Chromosome 6. Because we are diploid one Chromosome 6 is inherited from the dad and one is inherited from the mom. Thus, even siblings can bear different HLA molecules from one another.
Rock, K. L., Reits, E., & Neefjes, J. (2016). Present Yourself! By MHC Class I and MHC Class II Molecules. Trends in immunology, 37(11), 724–737. https://doi.org/10.1016/j.it.2016.08.010
Remember that epitopes are sections on non-self antigens that antibodies bind to. Therefore, if HLA molecules recognize these epitopes, it suggests that immune cells and antibodies should recognize them as well.
This shortened name for these epitopes use the same 3 starting amino acids (NQK), with the differentiating factor being where amino acids differ. For instance, the 8th amino acid from SARS-COV2’s epitope has a glutamine (Q), which is referenced by the Q8 addition to NQK. In contrast, seasonal coronaviruses carry an alanine (A) in the 8th position and therefore has the A8 naming.






Isn't this the same allelle implicated in increased risk of multiple sclerosis?
That:
"Note that this study took place in early 2020 up until April 2021- prior to any vaccine rollout."
is not right, here little bit more about the timing:
https://www.bbc.com/news/world-us-canada-55305720
the rollout started on 14 December 2020. While watching every single day, how many more got injected, I was TERRIFIED, that so many believed all the lies.
In the epitope story, the two substitutions by non-charged residues alanine and glutamine can't change the electrostatic interaction..