A Follow-up on the East Palestine Train Derailment
Some corrections/context on vinyl chloride, and is HCl the primary toxin released from the controlled fire?
Cover image from Inside Edition
Edit: Images were re-added. Please let me know if an “Image Not Found” box still appears on your end.
Yesterday I wrote a post on the train derailment that occurred in Ohio, noting in particular the toxicity of vinyl chloride as a carcinogen as well as the possible dangers of phosgene— a product of VC combustion.
In the comments Pete Lincoln pointed me to an article he recently wrote about the incident which added a lot more context, as well as some things I overlooked in my post from yesterday. It’s a very thorough article that collects from a lot of news outlets, so I recommend people read it.
So this post will add some corrections and additional context to the situation.
More on Vinyl Chloride and a Comparison to 2012 Paulsboro Incident
I discussed one of the main toxicities of VC, which was the possibility of liver cancers such as hepatocellular carcinoma (HCC) as well as angiosarcoma of the liver (ASL).
However, the risk of cancer has generally been associated with prolonged, chronic exposure to VC, such as what is seen with manufacturers who are exposed to VC on a daily basis.
Now, this doesn’t mean that there won’t be a risk of cancer in the immediate area, but that acute effects from VC may be more likely.
But given the evacuation in the days prior the level of VC exposure would be highly varied.
It’s hard to gauge the actual extent of exposure to East Palestine locals, but here the incident in Paulsboro, New Jersey may provide a comparison.
To that, one thing I overlooked was the density of VC. Although VC is volatile and will likely form gas at ambient temperatures, it also is more dense than air and will actually carpet the ground with a fog-like appearance*.
*Note: After looking into the information more I believe my following remarks on the VC fog may be heavily overblow. VC is generally colorless, and although it is more dense than air the fog-like appearance seen in the Paulsboro incident may be due to VC leaking into the river. Although VC is not water soluble, the interaction with water readily vaporizes VC, and thus may be the reason for the blanketed fog over the local environment. Given these caveats I want to raise some skepticism for the proceeding information, which I decided to leave in for posterity. However, if it raises more confusion than contextualization please let me know and I will make an edit.
This can be seen in one of the photos on the NOAA’s website for the 2012 Paulsboro, New Jersey train derailment, which appear to have been taken by a local resident:

As well as in this perspective from the accident report made by the National Transportation Safety Board which Pete Lincoln cites in his article.
It’s hard to determine the scope of this VC fog, and if it had made its way into the neighboring environment, although an article linked by Pete Lincoln notes that eye witnesses saw a large cloud, suggesting that it may have made its way to locals.
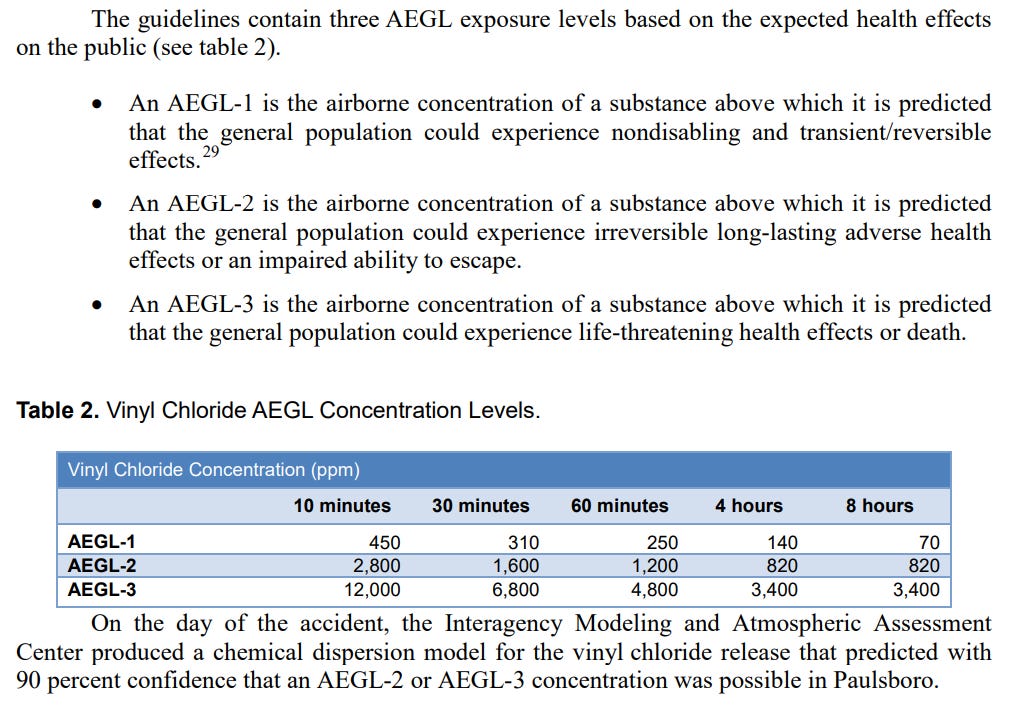
The accident report goes on to include a model of VC exposure that may have been possible for locals:
Although modeling data has some issues, the immediate environment was tested for volatile organic compounds (VOCs) which detected more than 500 ppm of VOCs with the assumption that much of these VOCs were from VC derived from the train leak (emphasis mine):
About 6 hours into the incident, the fire chief had yet to relocate the ICP to a safe location and failed to establish PPE requirements for the accident scene, despite the availability of air monitoring data that should have prompted these actions. At 8:34 a.m., a Paulsboro Refining Company air monitoring team began testing for volatile organic compounds (VOC) such as vinyl chloride.36 The air monitoring team’s certified industrial hygienist told NTSB investigators that while attempting to calibrate air monitoring instruments near the initial ICP situated next to the accident scene, their meters detected high atmospheric concentrations of a volatile compound. He informed those assembled at the ICP that the team had measured VOC concentrations in excess of 500 ppm and that the permissible exposure limit for vinyl chloride was only 1 ppm.37 The team reported that the air monitoring results indicated the ICP was located in a hazardous atmosphere. The highest level of vinyl chloride detected was at 1,444 ppm, far exceeding the AEGL-2 concentrations for a 60-minute public exposure.
In addition, the accident report provides a survey taken in 2014 asking residents who evacuated, as well as those who didn’t evacuate Paulsboro what they experienced after the incident. Note that the time discrepancy brings into question the reliability of said surveys, but it’s one of the only available surveys available aside from the hospital records:
A high percentage of participants in the two surveys reported smelling or tasting an unusual odor in the air during the incident. The most commonly reported symptoms were headache, upper respiratory symptoms, and coughing. Other common symptoms included: neurological symptoms (predominantly dizziness or lightheadedness), eye irritation, and difficulty breathing. The symptoms that were more commonly reported are consistent with what is known to occur from exposure to vinyl chloride.31 Symptoms were most commonly reported from evacuated areas and the area within 1 block of evacuated areas, and were least frequent in areas farther than 3,500 feet from the accident location.
Taken altogether, the picture suggests that most residents of Paulsboro may have been exposed to several hundred ppm of VC in the immediate vicinity, with higher levels possible if we consider the numbers provided by the CDC1.
Now, if we compare it to the current predicament in Ohio it doesn’t appear to have the same similarities.
When reviewing some of the images from the Ohio train derailment there doesn’t appear to be the same fog-like appearance around the trains (which may not actually mean much in the end), and the rush to create a controlled fire (which didn’t occur in Paulsboro) would suggest that much of the VC may have been combusted and the exposure rate may be lower than what had occurred in Paulsboro.
Again, this doesn’t mean that locals were not exposed to VC because of the train derailment, but it would at least suggest that exposure to VC was likely to be much lower in Ohio compared to Paulsboro.
How much less is the VC exposure in Ohio? This is difficult to determine without any quantification being conducted. As of now, the results of indoor and outdoor testing are assumed to be released some time in the near future. However, trust in the agency conducting the testing has raised some concerns whether proper tests are being conducted or if information is being withheld.
But as of now the Paulsboro incident may be the best comparison to the current incident in Ohio. The high dose of exposure for VC symptoms would need to corroborate with information from locals before the controlled fire to suggest how much exposure has actually occurred to locals. As of now it appears that many measures were taken days after the fire which wouldn’t provide accurate representative data.
Phosgene or Hydrogen Chloride as the more Toxic Product?
My post focused on phosgene given the report from Newsweek. However, upon closer examination it would appear that the concerns over phosgene may be a red herring stirred my mainstream press wanting to make allusions to chemical warfare agents.
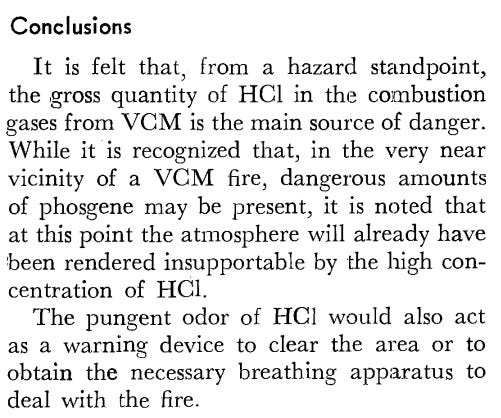
This is helped by Pete Lincoln’s citation of a study looking at products of VC combustion, which noted that phosgene actually makes up a very low number of the products (emphasis mine):
By means of a variety of analytical techniques, the combustion profile of vinyl chloride monomer (VCM) has been determined. This profile includes flame temperatures, soot content, and a combustion gas analysis. Depending on the amount of VCM-air premixing prior to combustion, the temperature of a VCM flame ranges from 950° to 1466°C. Similarly, the soot or unburned carbon content of a VCM flame varies from 3 to 6 weight percent. An analysis of the combustion gases from VCM reveal the following composition: HC1 27,000 ppm; CO2 58,100 ppm; CO 9500 ppm; phosgene 40 ppm; and VCM trace. From a hazard standpoint, the gross quantity of hydrogen chloride is the main source of danger in a VCM fire.
I actually noticed this study in my search but decided to overlook it. I probably should have actually read it as it suggests that the main toxin is the HCl produced by combustion of VC.
It’s important to note that products from combustion are heavily dependent upon the composition of the air, as well as other factors. However, the review from O’hara, et al.2 concludes with the following remarks with respect to HCl from VC combustion:
Hydrogen chloride is general found in its anhydrous form or as an acid in aqueous solution forming hydrochloric acid via the hydrogen and chloride ions it forms.
Because of its high capacity to act as an acid exposure to hydrogen chloride general leads to protonation of cellular structures and other issues, but these effects are usually offset by the buffering capacity of mucous membranes which may contain ammonia and other basis that can absorb the acidity.
Strangely, not much research has been done into looking at the toxicity of hydrogen chloride on humans, possibly due to the concerns over acute toxicity.
A case report from Faria, et al,3 in which an individual was exposed to hydrogen chloride in cleaning products noted some of the harms of exposure in the Discussion:
Inhaled HCl may cause functional and morphologic respiratory tract injuries, depending on the exposure concentration and duration. The maximum bearable concentration in prolonged exposure of humans was reported as 10ppm; however, 10-50ppm for an exposure of a few hours is tolerable. 8 Respiratory tract effects in laboratory animals range from mild to moderate irritation at low concentrations (less than 100ppm) to nasal lesions at moderate concentrations (100-500 ppm) and pulmonary damage at high concentrations (greater than 500ppm), even with fairly short exposure periods. The concentration of 1000ppm and above cause unconsciousness, followed by death in thirty minutes to one hour. 5 , 8
Because of its high-water solubility, most of the inhaled HCl gas is absorbed and neutralized by ammonia gas in the upper respiratory tract, explaining the occurrence of delayed respiratory irritation signs. 6 , 8 , 9 , 13 In fact, little is known about the acid-base buffering capacity of respiratory mucous membranes and tissues. 8
So even low-dose, acute exposure to HCl can lead to serious and persistent complications, and may lead to pulmonary edema as well as other serious respiratory issues.
The CDC also notes very low inhalation exposure to HCl and possible symptoms:
Hydrogen chloride gas is intensely irritating to the mucous membranes of the nose, throat, and respiratory tract. Brief exposure to 35 ppm causes throat irritation, and levels of 50 to 100 ppm are barely tolerable for 1 hour. The greatest impact is on the upper respiratory tract; exposure to high concentrations can rapidly lead to swelling and spasm of the throat and suffocation.
Most seriously exposed persons have immediate onset of rapid breathing, blue coloring of the skin, and narrowing of the bronchioles. Patients who have massive exposures may develop an accumulation of fluid in the lungs.
But again, how do we know specifically that the residents of East Palestine may have been exposed to HCl over phosgene? It’s likely all in the smell.
Phosgene apparently gives off a musky odor akin to wet hay. HCl, on the other hand, provides the all-too familiar smell of bleach and chlorine that you find in pools and other cleaning products.
The CDC notes that around half of the people exposed to 5 ppm of HCl can detect the pungent smell, and for many the smell is used as a sign to evacuate from the environment, so it doesn’t take much to notice it.
So any evidence of “bleach-like” smells may point to HCl poisoning.
Yesterday a story reported in Yahoo! News from The Independent told of a family who supposedly had respiratory infections in the days following the train derailment. There’s not much in explaining what this respiratory infection is, but the story provides a key detail to the smell in the local environment:
A couple and their three-year-old child are suffering from upper respiratory infections in the wake of the toxic train derailment in East Palestine, Ohio.
Local residents, Chris and Jamie Wallace, and their toddler, went to hospital with breathing issues which they said developed after the train crash.
“I knew something was different when we left town and there was that chemical smell in your nose, as if you were in the bathroom cleaning with bleach and you walk out and you still smell that bleach in your nose,” Jamie Wallace told NewsNation on Tuesday.
Remember that none of the other chemicals identified so far appear to be chlorinated organic compounds, and so the source of the chlorine had to have come from VC, which when combusted produced HCl and thus contributed to the bleach-like smell in the air.
Unfortunately, the story doesn’t tell us exactly when the Wallaces evacuated the area.
However, a local news report from February 6th noted that a strong bleach smell was noticed in the area, which was downplayed as being “safe” for the locals:

The Ohio EPA and U.S. EPA completed air sampling throughout Mahoning County and read no reportable contamination in the air.
Officials with Boardman are “strongly recommending” that residents stay indoors, though more as a precautionary measure.
“All the special monitoring is not picking up any dangerous readings at all. Nothing at all. All we have is an odor. Yes it is unpleasant to say the least. But that is all it is. Not dangerous at all. We will continue the recommended shelter in place for peace of mind over night and resume normal activity in the morning,” says Boardman fire Chief Mark Pitzer.
Strange that such proclamations were made, but what’s also interesting is the fact that no supposed contamination was measured.
Remember that HCl is not a volatile organic compound because, well, it contains no carbon and therefore is not organic. This raises questions if the tests for VOCs used could accurately measure HCl or if other tests were conducted to check for this agent.
Concerns for Locals, but not Downstream?
But again, as noted in the previous post, it’s possible that plenty of other agents, including ones produced from the controlled fire may be contributing to the environmental damage and toxic exposure to locals.
As to the wider environment more evidence would be needed to see what effects this spill has on downstream regions. As Pete Lincoln notes in his post, and as corroborated by news sources the Ohio River is considered the most polluted river in the US, with a news report from 2021 noting that the River received that moniker for the 7th year in a row, so it’s not as if the river is not already heavily contaminated (what’s a few drops of butyl acrylate to round out the pollution?)
The river has been the site of chemical dumps for decades, and a few years ago the Delaware-based company DuPont was involved in a lawsuit in which a man alleged that a compound (Perfluorooctanoic Acid, or PFOA) used by the company to make Teflon was being dumped into the river and led to his testicular cancer.
The fact that the river has been used as a dumping ground for decades may provide some (maybe?) perspective on whether the release of chemicals will be detrimental in the long run.
In that regard, the bigger concern would be the fallout in East Palestine as locals have to deal with the lack of transparency about whether the immediate environment is actually safe.
Again, hopefully more information comes out in the proceeding days (Also, check out Pete Lincoln’s post!)
Substack is my main source of income and all support helps to support me in my daily life. If you enjoyed this post and other works please consider supporting me through a paid Substack subscription or through my Ko-fi. Any bit helps, and it encourages independent creators and journalists such as myself to provide work outside of the mainstream narrative.

As a frame of reference:
Inhalation is the primary route of exposure, and vinyl chloride is readily absorbed from the lungs. Its odor threshold is too high to provide an adequate warning of hazardous concentrations. The odor of vinyl chloride becomes detectable at around 3,000 ppm and the OSHA PEL is 1 ppm (8-hour TWA). Therefore, workers can be overexposed to vinyl chloride without being aware of its presence. A 5-minute exposure to airborne concentrations of 8,000 ppm can cause dizziness. As airborne levels increase to 20,000 ppm, effects can include drowsiness, loss of coordination, visual and auditory abnormalities, disorientation, nausea, headache, and burning or tingling of the extremities. Exposure to higher concentrations of vinyl chloride for longer durations can cause death, presumably due to central nervous system (CNS) and respiratory depression. The gas is heavier than air and can cause asphyxiation in poorly ventilated or enclosed spaces.
O'Mara, M. M., Crider, L. B., & Daniel, R. L. (1971). Combustion products from vinyl chloride monomer. American Industrial Hygiene Association journal, 32(3), 153–156. https://doi.org/10.1080/0002889718506429
Faria, V. S., da Silva, S. A. E. H. C., & Marchini, J. F. M. (2021). Reactive airways dysfunction syndrome following inhalation of hydrogen chloride vapor. Autopsy & case reports, 11, e2021266. https://doi.org/10.4322/acr.2021.266






So... after hitting "publish" the images I included aren't appearing on my end. Editing on my side also brings up the same issue so it seems like Substack did something wonky and just removed my images, so please let me know if any of you are also getting this issue as well and I'll correct the issue, if possible. 🤦♂️
Edit: Images have been re-added. Please let me know if you are able to see them or if there are still issues.
'Image not found' on email but they show here online