1 in 35 COVID vaccinees suffer heart damage?
A new study suggests a higher rate of heart damage than previously reported from the COVID vaccines, although there's a lot that seems to be problematic with this study.
Edit 7.30.2023: The article previously mentioned “a new myocarditis study”. The study does not relate to myocarditis in particular, and so the phrasing was changed to “a new myocardial injury study”.
Correction 7.30.2023: Previously I mentioned that troponin levels were also measured on Day 30. In reality, the follow-up mentioned by the researchers referred specifically to the day after, or Day 4 to be specific. Apologies for overlooking this. In some ways this actually makes the study worse since it suggests that there are only 2 data points rather than the previous 3 I assumed.
A new myocardial injury study1, reported on by both Alex Berenson and Dr. John Campbell, reports a rather alarming study finding, suggesting that people who receive the COVID vaccines may be experiencing more heart damage than previously thought.
There is still so much that has yet to be revealed about vaccine-related heart damage, and most evidence has shockingly come out at a snails pace. Because of this, the findings of this study would be rather damning of more widespread damage that has yet to be fully elucidated.
And yet at the same time this study isn’t exactly one that provides more details as seems to be reported. Although these findings appear alarming, there’s been some details missing in some of the analyses I have seen, including this “1 in 35” that the study reports.
More importantly, in looking into this study a bit more there’s a lot of things that have left me wanting. There’s a lot that was not looked at, and there’s some curious things when it comes to what was found.
Study Overview
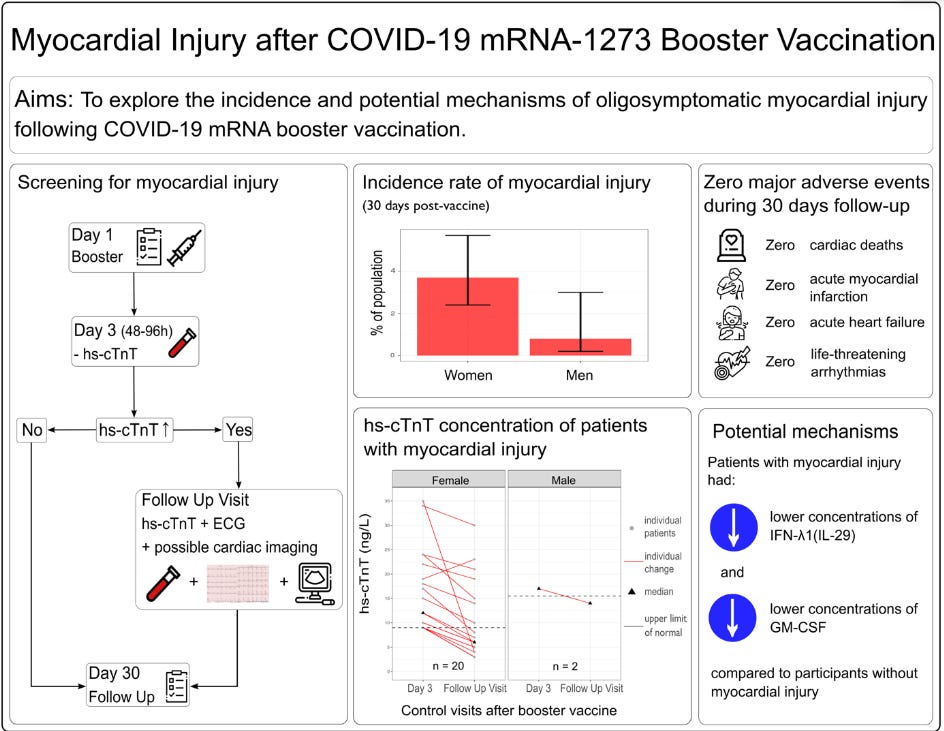
The study was rather straightforward, looking at over 835 employees at a hospital in Switzerland ( University Hospital Basel) who were going to receive the first booster of Moderna’s COVID vaccine.
Patients who consented had their troponin levels (indicated by hs-cTnT in the study) measured on Day 3. If troponin levels were above the upper-limit of normal (ULN) based on the individual’s sex they were ordered to not conduct strenuous exercise and had troponin levels measured on the following day (Day 4) in order to examine whether troponin levels increased, decreased, or stayed the same on Day 4 when compared to Day 3. Minimal increases (note this is relative) or no changes were considered to be possible underlying cardiovascular disease and these employees were excluded, leaving only 22 patients who appeared to show a Day 4 increase in troponin levels, suggesting a possible association between the vaccine and myocardial damage.
Patients were then measured on day 30 for troponin levels.
Note that this has been corrected, as the troponin levels were only measured on Day 3 and Day 4.
Note that although the researchers mention conducting imaging if necessary, it appears that no actual imaging was conducted at the 30-day follow up and was included as a possible limitation of this study:
Fifth, no CMR imaging was performed, as the amount of vaccine-induced cardiomyocyte injury in this study was below the expected limit of detection of CMR for late gadolinium enhancing myocardial lesions indicative of myocarditis (usually a hs-cTnT concentration of about 50-100ng/L).10,11
Now, I outlined all of this because it’s important to emphasize that any myocardial injury measured in this study was suspected based upon troponin levels alone. No other studies seemed to have taken place to indicate additional evidence of myocardial injury.
The researchers themselves used this definition of myocardial injury for this study:
…COVID-19 mRNA vaccine-associated myocardial injury was defined as acute dynamic hs-cTnT- elevation above the sex-specific 99th-perentile ULN ( 8.9 ng/L in women and 15.5 ng/L in men) on day 3, without evidence of an alternative cause, irrespective of symptoms, ECG, or cardiac imaging abnormalities. In the absence of a baseline hs-cTnT concentration immediately prior to the vaccination, strict criteria were applied in the adjudication of COVID-19 mRNA vaccine associated myocardial injury.
Also, note that no baseline troponin levels were reported for some of these employees, meaning that there’s limited comparative data between pre-booster troponin levels and post-booster troponin levels, so there’s some questions about what this acute elevation is on Day 3 when there weren’t any measures taken on Day 1 or pre-booster administration.
The authors provide some differential criteria in lieu of the lack of baseline levels, although I find this to be strange when the researchers could have just collected samples instead of setting up criteria:
In the absence of a baseline hs-cTnT concentration immediately prior to the vaccination, strict criteria were applied in the adjudication of COVID-19 mRNA vaccine associated myocardial injury. For the differentiation of acute COVID-19 mRNA vaccine-associated myocardial injury versus possible chronic preexisting myocardial injury, four criteria were used: first, the extent of the hs-cTnT elevation (the higher the elevation, the more likely acute), second, the extent in the change of hs-cTnT from day 3 to day 4 (the larger the change the more likely acute), third, previous hs-cTnT measurements if available in the medical history of the participants, and fourth, the likelihood for hs-cTnT elevation according to known causes of chronic myocardial injury, including age and preexisting cardiovascular diseases.
Note that even in these criteria there’s quite a bit of qualitative assessments being made. The difference between underlying cardiovascular disease and vaccine-related myocardial injury appears relative, with explanations for underlying cardiovascular disease being examples such as this where the changes in troponin aren’t fully explained (found in the Supplemental Table 4, Patient ID 4, Female):
Just mildly above the sex specific ULN and no relevant change between Day 3 vs Day 4, therefore chronic preexisting myocardial injury given the age of 52 is possible/likely
So maybe rates of myocardial damage may be higher than found in this study, or they may be lower. The problem is that such an arbitrary cutoff runs into issues of ambiguity with this data.
Note that the vaccine-related increase in troponin seemed to have occurred in an older cohort. The median age overall was around 37 whereas the median age for the vaccine-related myocardial injured cohort is around 46, suggesting that more than half of the participants in this group were above the age of 46.
If anything, one comment made in this study that is worth considering is the sex-difference in ULN troponin levels between males and females, with males having a cutoff nearly double that of females (8.9 ng/L vs 15.5 ng/L). This raises a serious issue of whether myocardial injury in women may be underreported if the wrong rubric is being used to measure for troponin.
Now, with all this being said let’s consider the initial hypothesis proposed by the researchers:
We hypothesized that COVID-19 mRNA-vaccine-associated myocardial injury following booster vaccination may be much more common, as symptoms may be unspecific, mild or even absent, escaping passive surveillance.
The question remains whether this study, based on measuring troponin levels alone, actually was able to answer this question. The problem with the researchers justification seems to be, unfortunately, based upon an apples to orange comparison between myocardial injury and reported cases of myocarditis:
First, our findings confirmed the study hypothesis. mRNA-1273 booster vaccination-associated elevation of markers of myocardial injury occurred in about one out of 35 persons (2.8%), a greater incidence than estimated in meta-analyses of hospitalized cases with myocarditis (estimated incidence 0.0035%) after the second vaccination.14,15
Note that there’s a misdirection here. The authors are comparing their reported cases of myocardial injury, a far broader diagnosis, to myocarditis. Remember that many cases of myocarditis are confirmed with either biopsies or cardiac imaging. In the case of this Buergin, et al. study it was argued that the cases were too mild to require an ECG and no imaging was conducted at follow-up, so we don’t really know how this cohort actually compares to prior studies. Because of this I don’t find it an appropriate comparison, especially given how broad and loose the metrics used were in the Buergin, et al. study.
Keep in mind that this study was intended to not impede daily functions, and in a paradoxical sense the study was designed to reduce the possibility that employees would not want to receive a booster if more tests were needed:
The following limitations should be considered when interpreting our findings. First, to interfere as little as possible with the motivation of the hospital staff to obtain the mRNA-1273 booster vaccination and its logistics, we restricted the study to blood draws after vaccination. Thus, baseline hs-cTnT values were not available.
So it looks like the researchers stifled their own study by not wanting to discourage vaccination, meaning no further testing and assessments were done.
As of now, readers can make what they want of this study. It leaves me wanting more than what was actually presented, and without additional details a lot of critical context remains missing.2
Reasons for elevated Troponin?
So it appears that the elevated troponin levels needed some more explaining. However, the researchers still seemed to look for biomarkers that may relate to an explanation for possible myocardial injuries.
The first choice was to look at autoimmunity, but in this case the researchers looked specifically at anti-interleukin 1 receptor antagonist (anti IL-1RA) antibodies.
IL-1RAs are an interesting group of proteins, which seem to act as antagonists for the inflammatory cytokine IL-1. As would be suspected, these receptor antagonists prevent IL-1 from binding to receptors and leading to an inflammatory response. Therefore, IL-1RAs act as inflammatory modulators, and may actually be cardioprotective.3
What’s interesting is that IL-1RA responses seem to be elevated post-mRNA vaccine use in mice, suggesting that this process may be a response to attenuate the inflammation caused by the vaccines.4
Following this logic, one can surmise that antibodies that target these receptor antagonists would block the attenuation of inflammation. Put another way, the presence of these autoantibodies would infer that inflammation would run rampant in these patients.
Although an interesting premise it appears that the vaccine-related troponin elevated cohort had lower rates of anti IL-1RA antibodies, although the lower levels don’t appear to be statistically significant. IL-1RA expression also appears comparable across the groups:
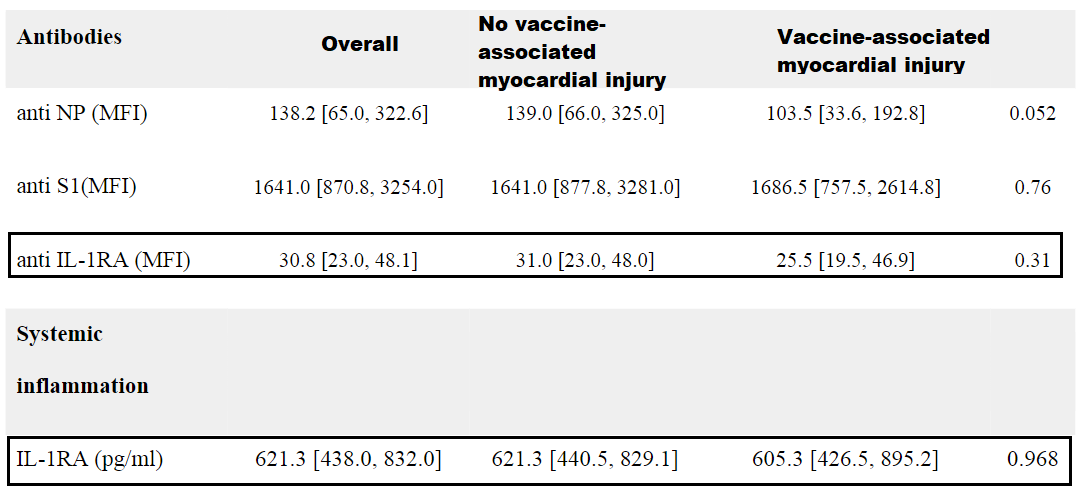
By all accounts, biomarkers for inflammation seem comparable across the 3 groups, so this may actually suggest that the myocardial injury in this case may not be myocarditis, although the lack of further assessments means we can’t make clear judgements in that regard.
I was curious why this autoantibody was chosen in particular. As we have noted previously, autoantibodies against adrenergic receptors have been documented in severe cases of COVID as well as in those who have experienced small fiber neuropathy post-vaccination.
It would make sense to check for other autoantibodies than just this one in particular.
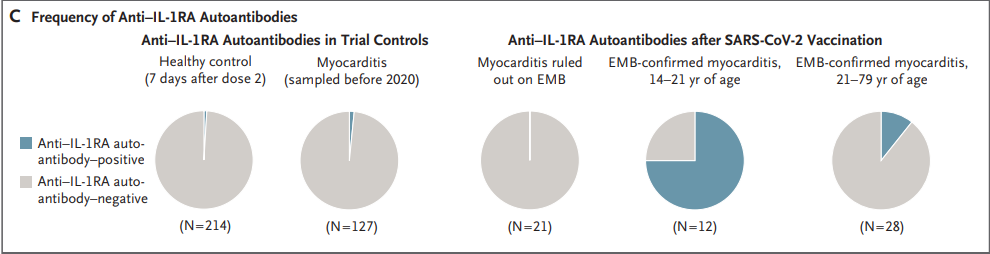
In doing a bit of perusing I came across this study from Thurner, et al.5 which noted that within this cohort of myocarditis patients anti IL-1RA antibodies were elevated in adolescents/young adults who had biopsy-confirmed myocarditis post-second vaccination. In contrast, older patients who had confirmed myocarditis had a lower bias towards anti IL-1RA antibodies:
Myocarditis was confirmed by biopsy in 40 of 61 patients (Fig. 1A). Among patients with histologically confirmed myocarditis, anti–IL-1RA antibodies were found in 9 of 12 patients (75%) younger than 21 years of age, as compared with 3 of 28 patients (11%) 21 years of age or older. Anti–IL-1RA antibodies were not detectable in the 21 patients in whom biopsy ruled out the diagnosis of myocarditis (Fig. 1B and 1C). IL-1RA antibody–positive patients with biopsy-confirmed myocarditis had an early onset of symptoms, which occurred mostly after receipt of the second vaccine dose, and a milder course of myocarditis than patients with biopsy-confirmed myocarditis but without anti–IL-1RA autoantibodies (Tables S1 through S6 and Figs. S1 through S6 in the Supplementary Appendix, available with the full text of this letter at NEJM.org).1,2
If valid, this study would at least suggest that looking at anti IL-1RA antibodies may not be a good predictor of myocarditis or myocardial injury in older patients (again, more evidence is needed to substantiate this claim). This study seemed to have been cited by Buergin, et al. (Citation #8), so it seems like they should have been aware of this discrepancy relative to the demographic makeup of the employees in their study.
Again, it raises a question of why this autoantibody was looked at in particular for the Buergin, et al. study. It at least suggests that autoantibody-related heart injury cannot be ruled out in this cohort since proper measures may not have been conducted, although inflammation doesn’t appear to be an explanation for these findings (due to low inflammatory biomarkers), so the exact reason for the elevated troponin isn’t provided.
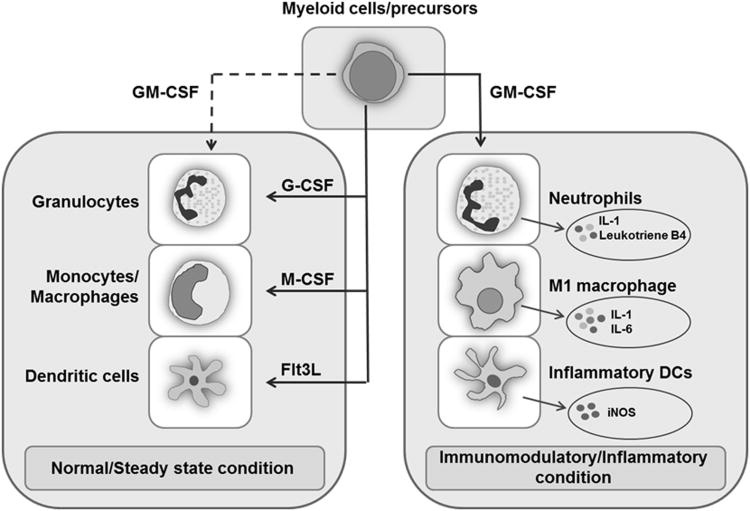
Now, the researchers note statistical differences in IFN-λ1(IL-29) and GM-CSF in the vaccine-injured groups compared to the other groups, noting that this may be something worth investigating in future studies.
They comment that these proteins may be related to innate antiviral properties, however this seems to be a bit of a simplification.
IFN-λ are type III interferons usually found within the epithelial or mucosal linings of the body, and in contrast to other interferons this group appears to operate both as an antimicrobial agent as well as a immunomodulator, regulating neutrophil activity and inflammatory responses.6
The same seems to go for granulocyte-macrophage colony stimulating factor (GM-CSF), which appear to aid in myeolopoiesis (formation of bone marrow cells) and regulation of inflammatory responses. GM-CSF appears to be a critical suppressor of autoimmunity.7
It’s interesting that these two factors seem to be reduced in this vaccine-injured cohort, although why they were reduced is not explained.
Maybe others can figure out the roles of these two factors. For now, this study unfortunately doesn’t go deep enough in assessing these biomarkers and determining what type of myocardial injury, if occurring, occurred in these individuals. Instead, it just suggests that troponin levels appear to be elevated, but not much else can be gleaned from this study.
However, hopefully this study further emphasizes the significance of looking at different factors, including age, sex, and dosage number when considering adverse reactions.
Substack is my main source of income and all support helps to support me in my daily life. If you enjoyed this post and other works please consider supporting me through a paid Substack subscription or through my Ko-fi. Any bit helps, and it encourages independent creators and journalists such as myself to provide work outside of the mainstream narrative.

Buergin, N., Lopez-Ayala, P., Hirsiger, J.R., Mueller, P., Median, D., Glarner, N., Rumora, K., Herrmann, T., Koechlin, L., Haaf, P., Rentsch, K., Battegay, M., Banderet, F., Berger, C.T. and Mueller, C. (2023), Sex-specific differences in myocardial injury incidence after COVID-19 mRNA-1273 Booster Vaccination. Eur J Heart Fail. Accepted Author Manuscript. https://doi.org/10.1002/ejhf.2978
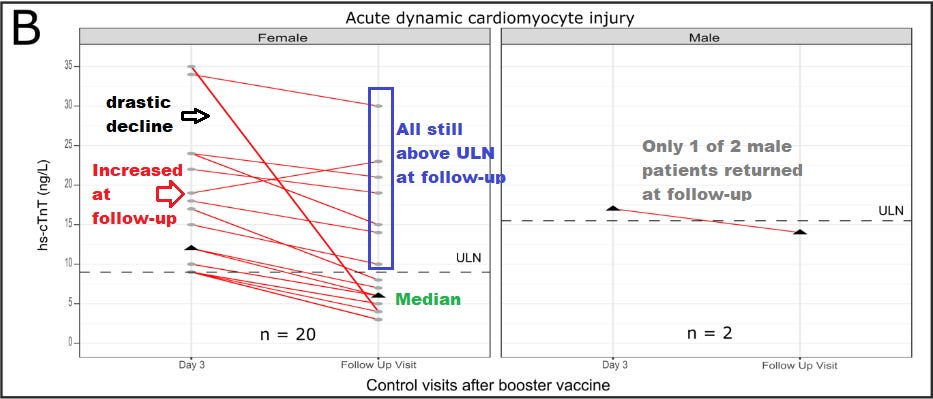
Consider the fact that one patient seemed to show a drastic decline in troponin levels during follow up, several patients still showed levels above the ULN, and one patient strangely appeared to show an increase. All of these findings didn’t seem to be further addressed in this study, so what exactly was the point of the follow-up if no additional interpretations were done for these people?
Figure 2 shown below:
Suzuki, K., Murtuza, B., Smolenski, R. T., Sammut, I. A., Suzuki, N., Kaneda, Y., & Yacoub, M. H. (2001). Overexpression of interleukin-1 receptor antagonist provides cardioprotection against ischemia-reperfusion injury associated with reduction in apoptosis. Circulation, 104(12 Suppl 1), . https://doi.org/10.1161/hc37t1.094871
Tahtinen, S., Tong, AJ., Himmels, P. et al. IL-1 and IL-1ra are key regulators of the inflammatory response to RNA vaccines. Nat Immunol 23, 532–542 (2022). https://doi.org/10.1038/s41590-022-01160-y
Thurner, L., Kessel, C., Fadle, N., Regitz, E., Seidel, F., Kindermann, I., Lohse, S., Kos, I., Tschöpe, C., Kheiroddin, P., Kiblboeck, D., Hoffmann, M. C., Bette, B., Carbon, G., Cetin, O., Preuss, K. D., Christofyllakis, K., Bittenbring, J. T., Pickardt, T., Fischer, Y., … Klingel, K. (2022). IL-1RA Antibodies in Myocarditis after SARS-CoV-2 Vaccination. The New England journal of medicine, 387(16), 1524–1527. https://doi.org/10.1056/NEJMc2205667
Zanoni, I., Granucci, F., & Broggi, A. (2017). Interferon (IFN)-λ Takes the Helm: Immunomodulatory Roles of Type III IFNs. Frontiers in immunology, 8, 1661. https://doi.org/10.3389/fimmu.2017.01661
Bhattacharya, P., Thiruppathi, M., Elshabrawy, H. A., Alharshawi, K., Kumar, P., & Prabhakar, B. S. (2015). GM-CSF: An immune modulatory cytokine that can suppress autoimmunity. Cytokine, 75(2), 261–271. https://doi.org/10.1016/j.cyto.2015.05.030




I wonder if the vagueness of this study, as you allude to, is due to a bias towards not discouraging booster uptake.
As they pointed out.
After all if those conducting the study don’t want to discourage booster uptake. Then they need a lot a wiggle room in data assessment.
Thank you for referencing your prior article discussing SFN. My dad (77) has had all the Moderna and at least one Pfizer because the Modernas made him feel horrible. I'm not sure when the latest one was, but he's recently developed numbness in his feet. A neurologist dismissed it as nothing, but now I'm wondering if he could have vaccine-induced SFN. Not that he or any of his drs would listen to me if I suggested it, sadly.