What I Learned Watching the Molnupiravir Committee Meeting (Review)
The Bureaucratic Process, and the Alarming Molnupiravir Signals
Well, I did it, I watched the ENTIRE 9-hour Committee Meeting for Molnupiravir from Tuesday!
OK, maybe we can remove the 2 hours of elevator music, but around 7 hours is a ridiculously long meeting regardless!
It was a slog to get through, but it really provided a look into the approval process of a drug. And frankly, it’s quite shocking, far more shocking than I thought it would be.
So I won’t bore people by posting all of my notes (I’ll provide a separate post), but instead I’ll point out the general process and some of the larger concerns brought up in the meeting. Any link in regard to the actual meeting will be a link to a selected timestamp so that my readers can see for themselves. Also, all of the slides were captured from the stream as the ones shown by Merck were not available.
The Bureaucratic Process in Action
Of course, like all formal procedures, the process was entirely slow, and unfortunately many of it was boring- I did not expect to hear the phrase “your mic is muted” so much!
Now, I never sat through one of these meetings in full. Usually I have seen clips of these things, and as we all know clips tend to exclude context. And I have done this myself when the first reports of the meeting came out and I quickly rushed to make judgements based on other people’s Twitter comments. It’s the reason why I thought it important to actually sit through and watch it in full; I couldn’t rely on other people’s summaries, which may be heavily biased.
To start the meeting, after the introduction by the FDA committee, Merck (the “Sponsor”) goes first and reports their findings. This was broken up into 3 parts.
Introduction and Mechanism of Action
This was pretty straightforward, so I won’t go into detail about it. Merck scientists just explained how the drug works in minimal detail. So the mutagenic effects were outlined. However, Merck also indicated spike protein mutations, although these mutations seemed random and they could not fully attribute them to Molnupiravir.
Nonclinical Studies (Timestamp)
Now, this is where it gets interesting. Nonclinical studies included both in vitro and in vivo assays (both cell cultures and animal models). Now, based on the press release the only information I had available at the time about nonclinical studies were both the Pig-A and Big Blue assays, both of which were intended to measure in vivo mutagenic effects of Molnupiravir. But here, they mentioned far more studies, all of which produced very alarming results.
Here’s a quick summary taken from the FDA’s Briefing Document in regards to the first few studies, although I will include a few important points that stuck out (emphasis mine):
Ames tests were conducted with the ester prodrug (MOV; EIDD-2801) and initial metabolite, NHC (EIDD-1931). EIDD-2801 was positive for mutagenicity in Escherichia colistrain WP2 uvrA and Salmonella typhimurium strain TA102, but negative in Salmonella typhimurium strains TA98, TA100, TA1535, and TA1537. EIDD-1931 was positive for mutagenicity in E. colistrain WP2 uvrA, but negative in S. typhimurium strains TA98, TA100, TA1535, and TA1537. EIDD-1931 was not tested with strain TA102. The in vivo and in vitro micronucleus assays showed negative results.
Note that other nucleoside analogs have been tested for genotoxicity and were positive in in vitro and/or in vivo assessments (see Table 10).
MOV was further evaluated in two established in vivo assays for mutagenicity: the Pig-a assay and the transgenic Big Blue® rat assay. The results of the Pig-a assay were “equivocal” as the assay showed a positive trend but results did not exceed the range of negative historical control values. In the transgenic Big Blue® rat model, the drug was evaluated for increased mutant frequency at the lambda cII transgene in liver and bone marrow. The assay result was negative for in vivo mutagenicity.
Ames Assay: The ames assay is an assay that uses bacteria to examine the mutagenic effects of a drug. This assay turned up positive for mutagenicity.
Human TK6 Assay: This was another in vitro assay, although this one utilized human cells. This assay turned up negative. However, Merck mentioned the results of the Zhou et. al. Study which indicated mutagenic results in a mammalian assay. In fact, the Zhou et. al. Study was referenced several times in the meeting as a concerning result, and so it was reassuring to see that many people were concerned with the results of this study.
Pig-a Assay: Now, this study stuck out, and for all the wrong reasons. The April Press Release of Merck was one of the only areas where this assay was mentioned, and the Press Release, in my perspective, made it seem that the results of this assay were positive:
Merck has conducted a comprehensive nonclinical program to characterize the safety profile of molnupiravir. This program included assays such as Big Blue and PIG-a which are designed to provide a robust measure of a drug or chemical’s ability to induce mutations in vivo. Animals were administered molnupiravir for longer and at higher doses (mg/Kg) than those employed in human studies. The totality of the data from these studies indicates that molnupiravir is not mutagenic or genotoxic in in vivo mammalian systems.
Ah, maybe it’s the phrase “totality of the evidence” that I should have looked out for, because as it turns out the Pig-a assay did not show great results. In fact, the results, as Merck indicated and as noted above, were “equivocal”, meaning that they could not tell whether it was positive or negative for mutagenicity. Immediately, I grew suspicious, as it seems the results of this assay were essentially swept under the rug.
And there seems to be evidence of that. As reported by both Merck, and later an FDA member, when a Pig-a assay result comes up “equivocal”, the expectation is to examine the data and see where the issue lies and try to correct it. But what did Merck do instead? They moved on to the Big Blue assay, which ended up showing a negative result for mutagenicity, which was reported and used to validate the argument that this drug was not a mutagen.
So how can it be that when one assay shows up with inconclusive results, and one shows up negative, the argument becomes to rely on the “preponderance of evidence”, such that the Big Blue assay was “good enough” to indicate lack of mutagenicity?
Fortunately, many of the committee members took Merck to task on this. It became one of the most highly contentious points in the meeting, including comments made by some members that questioned the way the study was conducted as well as the results.
For an even greater indictment, the panel later opened up the discussion to the public, with a former toxicologist calling out the toxicology studies, suggesting that they did not follow proper industry standards. One of the more egregious comments made suggested that the confidence cutoff in Merck’s Pig-a assay was set to 6 mutations/million base pairs whereas the typical upper limit cutoff is set to 1-3 mutations/million base pairs. By using a much higher threshold, Merck could argue that they did not see any significant mutagenicity, and even then the results were “equivocal” even though there seemed to be a trend in the data. To get a better account, the former toxicologist appears around the 05:22:00 timestamp.
But it doesn’t stop there. In fact, the results of the nonclinical studies became the largest areas of concern in the committee, and you’ll see why.
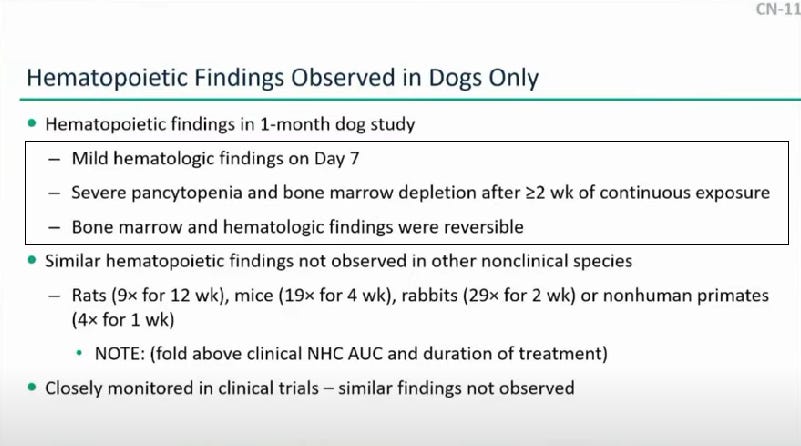
Hematopoetic Study in Dogs: This study was an in vivo study that examined the effects of Molnupiravir on blood biomarkers. Concerns arose that blood markers such as red and white blood cells, as well as bone marrow, were reduced over long term use of this drug. Remember to keep this in context; this study was conducted in dogs that were administered orders of magnitudes higher of Molnupiravir than humans over the course of a month. Nonetheless, these studies are used to extrapolate to clinical studies, and so they are used to point to possible human toxicity. Again, many members were concerned with these results.
Growth Plate Study: To add onto the hematopoetic study this study examined the effects of Molnupiravir on the growth plates of rats. Growth plates are areas inside bones where bone elongation occurs, and it’s an area where children may sometimes experience pain during puberty due to growth spurts. As they reach adulthood the growth plates begin to ossify and harden, and thus bone elongation ends. Therefore, growth plates are where rapid cell division occurs, and hindering this could lead to developmental issues. As noted, a rat study found that excessive use of the drug lead to increased growth plate thickness, suggesting that bone development was affected by the drug.
Although no children were evaluated in this study, this result, like the dog study, made many of the members concerned about possible toxicity in children, and considering the high-risk categorization provided by the CDC Molnupiravir was absolutely not considered for those under 18. As you will begin to see, the nonclinical studies led the members to suggest more and more restrictive use of Molnupiravir.
Embryofetal Development Study: Like the results of the Pig-a study, the results of this study caused a lot of controversy. In this study, pregnant rats were administered Molnupiravir, leading to fetal-lethality as well as teratogeniticy. At lower doses, both rats and rabbits exhibited weight loss.
This, of course, led to widespread worry about the use of this drug in pregnant women, but it didn’t stop there. Concerns later came up among members of the panel in regards to lactating women possibly passing the drug through breast milk, and even women who may want to become pregnant. Eventually, some of the members became vocal about the possible effects on spermatozoa; essentially any gamete was brought up as possibly experiencing mutagenicity. Remember that the clinical trials for Molnupiravir were highly exclusive. Men were not allowed to donate sperm a month after the last dose, people were not allowed to engage in sexual activities that may lead to pregnancy, and women were expected to use contraception.
Not only did the results of these studies cause concern in regards to pregnant women, now the concern arose about millions of patients taking this drug, experiencing gamete mutations, and possible passing on those mutations to their offspring. Although some rat studies did not show this occurring, this didn’t help to assuage the concerns of many committee members; the uncertainty was far too severe for many members to overlook.
Again, restrictions were considered, with members essentially arguing whether or not pregnant women should be provided this drug, with many stating “no” and a few suggesting only under severe circumstances. But with other concerns brought up, some community members even suggested this drug be limited to high-risk groups over the age of 60 and unvaccinated, an extremely limited patient demographic. This creates a strange conundrum; a drug that’s toxicity is so uncertain that its use is likely limited to a very narrow group. At this point the real argument is if this drug would even be worth approving with such a limited scope of usage.
The results of these nonclinical studies were shocking. In no way did it seem to serve to help Molnupiravir’s image. Actually, I would argue that it made many people far more concerned than they originally were. Finding out that animal studies showed teratogenicity (hello thalidomide!), that children may have their growth stunted, or that pregnant women or anyone who would want to become pregnant may be at risk of the mutagenicity served only to show the dangers and uncertainty of Molnupiravir more than it does to prove its safety and efficacy.
Clinical Trials
I’ll try to limit my response to this section, and I’ll include a separate post of my notes for those who may want to find time stamps of information.
To summarize, Merck researchers found obesity to be one of the highest risk factors for severe COVID, particularly in those with diabetes. This is something we have known long and well since the pandemic began. Here, we see more evidence of something that is both so obvious and yet so within people’s capabilities to change.
At this point we are fully aware of the lack of media information on diet, exercise, and supplementation to curb severe COVID. For a drug with such a high/uncertain toxicity profile to be the one to help save people from dying really reeks of the sad state modernity has left us in.
A few other things to note:
Molnupiravir seemed to have the greatest benefit in those over the age of 60; one of the reasons why this group was considered as a treatment group
the drug did not seem to have a large effect on diabetes patients
Adverse events of nasea/vomiting seemed to have appeared in the treatment group (unless I am mistaken), which adds an interesting comparison to the weight loss seen in the animal studies, although members of the committee did not bring that up (this slide was brushed over)
There was reduced efficacy in Delta compared to other variants. Although not discussed, I would argue that this may stem from Delta’s quicker infection rate, highlighting the importance of early therapy and timing of drug administration.
But this leads to the most alarming data. This was something I mentioned in my prior post where the final results of the MOVe-OUT study absolutely did not make sense; how it could it be that the data in the second half of the study differed so greatly from that of the first half? Luckily, this sentiment was shared by many of the committee members, and it led to some of the greatest skepticism about the studies.
When members were able to ask about these drastically differing results, I expected Merck to at least come up with some rational basis for this. Maybe it was based on the region of the study, the variant, or maybe comorbidities. What was the response by Merck?
They didn’t know why the results were so different.
Yes, Merck absolutely could not determine why the second half of the results were so drastically different! They mentioned the second half of patients were predominately Delta, and Molnupiravir did not seem very effective in this group. But that wouldn’t explain the drastic change in the placebo group. Merck also mentioned that this group had higher antibody presence, although they could not determine if it was due to prior infection or because of the emergence of antibodies from the current infection. So Merck couldn’t even figure out what caused this drastic change, and many members of the committee shared this sentiment; they did not feel comfortable with the inconsistency in the data.
Merck’s representative even mentioned that they expected a “regression to the mean”, suggesting that the second half of the study’s data should seem proportional to the first.
The only way I see it (and some of the committee members may have alluded to this) is that if Part I of the MOVe-OUT trial, if it met the efficacy confidence interval, then Merck would be able to seek EUA approval.
What does this mean? Well, if your company would like to seek approval and it only needs part of the data, wouldn’t you want to “fluff” up the data that is needed? Merck’s own representative mentioned that the final results were “important, but supplemental”, and that the efficacy confidence was based solely on the interim data.
Personally, there is no way I could see this and think that Merck was operating in good conscious. Such an extreme disparity in data can’t be seen as nothing more than evidence of something nefarious at play here. As I saw it, that data would have been enough for me to consider voting NO on approval. Unfortunately, I don’t have that option, and even though many of the members agreed it seemed there were enough that were able to overlook this piece, even though a majority of them did not feel favorable towards such inconsistent data.
Batting for the Other Team?
Again, I really did not understand how the committee process typically goes when I decided to watch this. I certainly did not expect the FDA to go after Merck, and I especially did not expect them to essentially rehash what was stated by Merck.
And yet here it happened; a federal institution, using the information provided by the “Sponsor”, essentially restated the evidence provided by Merck. Now maybe this is typical of the process, but either way that doesn’t negate the fact that this felt like a bold-face indication of institutional capture.
You’re telling me that the FDA did not do their own tests, but instead relied on the data presented by Merck?
And this happened quite often. If you listen to the open question portion, many questions were brought up about the Pig-a assay and the “equivocal” results. So how did a member of the FDA respond to the results? Well, they apparent passed the “eyeball test”.
Yes, someone who is in charge of one of the most vital regulatory bodies in our country essentially said that Merck’s results looked good enough to them, and admitted to not have conducted their own toxicology studies. Now, the FDA conducting their own studies is not a concern, but it is extremely disheartening that members of the FDA put so much weight onto the results provided by a pharmaceutical company and haven’t seemed to approach the results with a higher level of skepticism.
I can’t consider this in any way to be good science, especially when many others have questioned the validity of these studies!
There are other examples of this sprinkled in my notes, but this stood out to me the most. To think that the FDA would have the audacity to act as if the highly questionable evidence, as presented by Merck, should be considered with such a level of legitimacy speaks of nothing more than a cooperation between both pharmaceutical companies and federal institutions.
This revelation was stunning; the efficacy data was spotty at best and yet to consider it with such regard, instead of considering it with the air of skepticism that it should have been given comes off horrendously biased in favor of Molnupiravir, even with all of the inconsistent data and concerns of mutagenicity.
THE VOTES ARE IN
As I am running out of space, I will refer people to my notes for a breakdown of how the committee members voted and why.
Instead, I will point to something absolutely shocking. Right when the committee was asked to vote, they were told they could ask questions about the voting process.
One member, instead, asked a startling question: did they ever really prove the drug was effective.
After all this talk about the mutagenicity, the evidence suggesting that there is an adverse effect on bone plates, developing fetuses, pregnant women, and anyone expecting to become pregnant, members of the committee were STILL UNSURE if the drug had any benefits!
If committee members couldn’t even assess the effectiveness of Molnupiravir, how could they expect to take this drug to market?!
At that point I was honestly done. I couldn’t believe that after all of the safety signals, with members unsure of whether the drug actually worked, that they still found it acceptable to push this drug to market!
And yet they voted, with hesitancy, to do so. It made me think. The Kyle Rittenhouse trial was based off of the idea of “without a reasonable doubt”; if the prosecution could find no obvious evidence that Kyle was guilty, or if there was even the slightest hint of doubt, he could not be prosecuted.
Yet here, a drug that has the likelihood to affect the lives of millions of people worldwide, only needed to slide through on the thinnest margins.
Shocking, really, to think that the health and safety of millions of Americans could be put into the hands of such a few people, all with the hope that things will work out in the end.
I have lamented the fact that the Precautionary Principle has been so rarely evoked during the pandemic, and right when we reached a point where many should find a reason to evoke it, it was put to the wayside.
Watching the entire meeting seemed like a fruitless endeavor, but I honestly learned a lot from it. I learned how many of the voting procedures operate, seemingly against the benefits of the American people. I’ve also seen what appears to be blatant institutional capture, of those within federal agencies falling back upon the evidence supplied by Big Pharma rather than coming at it with a great deal of skepticism.
This endeavor has left me disheartened, because it really showed that all of the talks of seediness conducted by our institutions are not only ubiquitous, but apparent, as this committee meeting was allowed to air to the public.
At the time of me finishing this, I have yet to hear whether the FDA has approved Molnupiravir. This vote only adds a suggestion to approve, not to actually approve of the drug. The votes are intended to act as a recommendation.
If the FDA is to show any evidence that they have not been captured by pharmaceutical industries, they should be well-advised to vote against Molnupiravir’s approval. Otherwise, we may be putting the lives of millions of Americans, and millions of people worldwide in danger.
We’ll have to wait for the approval to see.
Note: There’s a lot to discuss with some of the other ideas brought up in the meeting. Some are absolutely ridiculous! They’re detailed in my notes, but I plan on writing a separate post outlining some of these additional info such as the concern over escape mutations, these weird mitigation strategies if they were to arise, and other weird, quite frankly out of touch comments about the use of Molnupiravir. In the meantime, I’ll provide a recap from the voting procedure as outlined by Dr. Baden and indicated in my notes.
Recap: 13 Yes, 10 No
Some who think absolutely no
some inclined for yes
most in middle
big questions are how to interpret efficacy
“Yes” Side
efficacy outweighed risks and heterogeneity of data
post-exposure monitoring needed
should be limited to high-risk individuals
many concerns over pregnancy
very few alternatives available
concerns about genotoxicity, mutagenesis weigh less because of lack of alternatives and reduction in mortality
role in high-risk patients such as transplant recipients need to be addressed
have to look into those who are unvaxxed but immunocompromised
No Side
too many uncertainties
efficacy data is “wobbly”
inconsistencies with data in 1st half and 2nd half of MOVe-OUT trial
genotoxicity, mutagenicity, impact on viral replication and viral escape all concerns-data lacking to inform those risks
risks in context of marginal benefit did not seem appropriate
Thank you for reading my newsletter. If you enjoy my articles please consider becoming a free subscriber in order to receive notifications.
And share with others who may find these newsletters interesting.
Also, please consider becoming a paid member. The research and work put into these articles takes many hours and being a paid subscriber allows me to continue to do this full time.
Supplemental Material
FDA Briefing Document. Taken from https://www.fda.gov/media/154418/download
FDA Committee Meeting Slides. Taken from https://www.fda.gov/media/154473/download








Thank you very much for watching that whole committee meeting and giving us insight into how it works. You are awesome! The regulatory capture is obvious from the outside, and you have shown how it works on the inside. 👍🏼
It is indeed disheartening to see how sausages are made. Although I did not have a chance to watch this one, I did watch the vaccine committee consider the Pfizer shots for 5-11 year olds in its entirety. and all I could do was shake my head.
BTW, I don't think the FDA ever does its own research or studies. It is up to the company seeking approval for the product to submit such data. I suppose the FDA might also take independent research into account, but it would be rather unusual for independent research to exist when it comes to a new drug.