Omicron has lost Binding Affinity to Human ACEII Receptors, but Gained Greater Cell-to-Cell Viral Transmission
Basic viral evolutionary principles suggest that as viruses continuously circulate within a population and become endemic, they are expected to become more transmissible but less virulent. Although it’s a rule of thumb, it tends to ring true for many of the seasonal colds and flu viruses we come into contact with annually.
There’s many facets that attribute to a virus’ more infectious abilities, but mutations in antigens responsible for binding to host receptors tend to play the largest role. In that regard, there’s an association between an antigens’ mutations and binding affinity to host receptors (in the case of SARS-COV2 that would be the virus’ spike protein becoming tailored towards human ACEII). With how infectious Omicron is, one would expect spike protein mutations in favor of ACEII binding. But if we look at some of the evidence, we actually see something quite contradictory.
In a study (Zeng et. al.) researchers transfected cell lines to uniquely express one of each SARS-COV2 common spike protein variants. Soluble ACEII receptors were then introduced and binding between the cell line and human ACEII receptors were measured. Contrary to what would be expected, Omicron showed reduced binding towards human ACEII compared to prior variants.
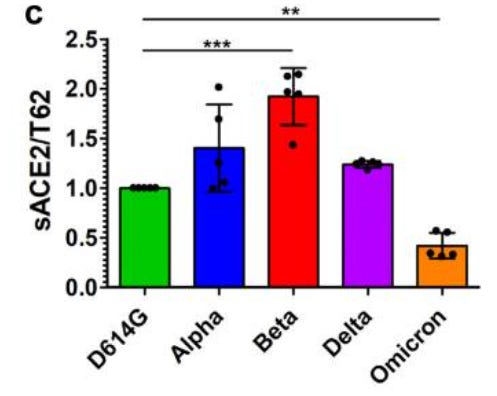
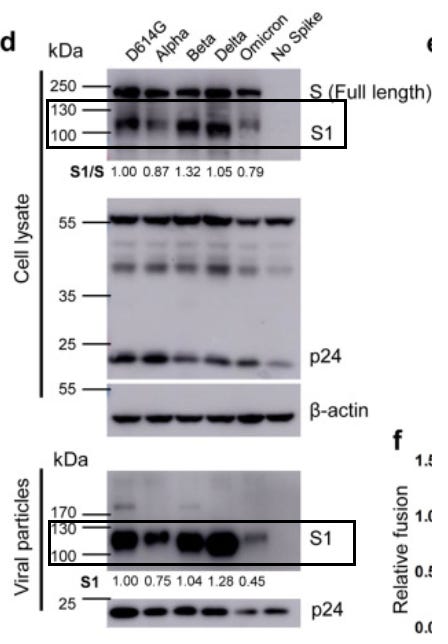
Adding to this information, the researchers also measured how readily the furin cleavage site of these variants can be cleaved by serine protease TMPRSS2. For the sake of brevity we will refer to this protease just as serine protease. The furin cleavage site rests in between the S1 and S2 subunits of SARS-COV2’s spike protein, with the S1 subunit being responsible for viral binding to host cell receptors while the S2 subunit is responsible for viral attachment and entry into host cells. Therefore, variants that have evolved to have their furin cleavage site more readily cleaved should be capable of greater entry into host cells and thus be more infectious and possibly more virulent.
Once again, results of this in vitro assay run counter to this theory with respect to Omicron, as it appears that the mutations in Omicron’s furin cleavage site show reduced spike protein cleavage activity. It’s worth noting that Delta shows very clear cleavage of the subunits, lending credence to the mutations in the furin cleavage site playing a major role in Delta’s increased transmission over prior variants.
So far we have evidence of the information working against Omicron’s evident increased transmissibility. Not only does it exhibit reduced binding to human ACEII receptors, it also appears to have reduced cleavage capacity of the S1 and S2 subunits. So where does Omicron’s increased transmission come from?
The researchers conducted another study, indicating that Omicron’s higher infectivity may stem from cell-to-cell viral transmission. In general, viruses spread through two pathways; they can either be released into the host’s extracellular matrix (also called cell-free transmission) where the virus roams freely until it comes into contact with another host cell to infect, or the virus spreads through the contact of one infected host cell to neighboring cells leading to a tangential form of infection (cell-to-cell transmission). Although it seems like a minor detail, a virus’ capability to spread in between host cells rather than through circulation creates many problems with respect to therapeutics and immune response, as many viruses are able to escape being targeted by both antivirals and the host immune system through utilizing the cell-to-cell route of transmission.
Indeed, cell-to-cell transmission of SARS-COV2 provides a plethora of concerns. With respect to Omicron, even though Omicron has reduced ACEII binding affinity, as well as reduced capabilities of producing cell-to-cell fusion (also called syncytia formation) it appears to have greater cell-to-cell viral transmission compared to prior variants.
It’s important to make a clear distinction here; cell-to-cell fusion refers to a virus’ ability to fuse neighboring cells into a large, multicellular mass (syncytia) whereas cell-to-cell transmission refers to a virus’ ability to infect neighboring cells. Both go hand-in-hand, although here we see that Omicron has higher transmission with reduced fusion.

This presents with a new problem. If Omicron’s infectivity depends predominately on cell-to-cell transmission there should be concern over the possibility of both immune escape and reduced antiviral effectiveness. It may also play a role in the greater reduction in monoclonal antibody neutralization, although this depends wholly on the type of assay being conducted. Questions should be raised as to whether this ability to escape immune function may play a role in preventing adequate vaccination against Omicron if the antibodies produced are not capable of targeting the variant due a possible mechanism of viral transmission.
It’s also worth noting that these in vitro assay results of reduced ACEII binding contradict the computer modeling done by Chen et. al, which can likely be attributed to the difference in methodology. Remember that computer modeling is heavily biased towards the variables provided by the researchers which could influence the data collected.
Also, the results here were all conducted in vitro, and considerations must be made to account for the fact that these won’t take into account the actual dynamics of viral binding, fusion, and transmission in humans.
However, these studies do provide some insights into a few key points:
Omicron appears to have reduced ACEII binding affinity compared to prior variants. This is likely to attribute to Omicron’s reduced virulence.
Serine protease activity against Omicron’s furin cleavage site appears to be reduced, again suggesting a possible reduction in virulence and infectivity of this variant.
Paradoxically, Omicron appears to have a robust increase in cell-to-cell viral transmission, which appears to be the likely culprit for Omicron’s high transmissibility and infectivity compared to prior variants.
Here’s a summary provided by the researchers (emphasis mine):
One surprising finding is that, distinct from Alpha, Beta, and Delta, the Omicron spike exhibited reduced binding to soluble ACE2, which likely accounts for, at least in part, its lower cell-cell fusion efficiency. This may indicate a fitness cost following an accumulation of RBD mutations while under selective pressure for nAb escape. Additionally, reduced cell-cell fusion would reduce cytotoxicity and could contribute to a lower virulence for the Omicron variant, which has been tentatively reported by anecdotal evidence23…
Indeed, the Omicron variant exhibited enhanced cell-to-cell transmission, which would facilitate virus spread. Cell-to-cell transmission is commonly used by many viruses, including SARS-CoV-2, and is a highly efficient mechanism of virus spread within a host20,26,27. Enhanced cell-to-cell transmission may help compensate for other observed defects in the Omicron S protein, such as reduced ACE2 binding and fusogenicity. Notably, cell-to-cell transmission of SARS-CoV-2 does not absolutely require ACE2, and extended cell-cell fusion by its spike impairs cell-to-cell transmission20. Additionally, cell-to-cell transmission is resistant to neutralizing antibodies, implicating another potential mechanism of Omicron immune evasion20,26,27.
Overall, Omicron provides us with a strange new beast. It’s behavior seems to run counter to prior variants, which is not something one would expect. It begs the question as to why Omicron is so different compared to other variants, and what questions it evokes as to how such a drastic change in infectivity has occurred.
Part III will look into Omicron’s antibody escape capabilities, and what that would mean for those who were previously infected or received 2 doses of the vaccine.




Thanks for this timely piece! Extremely interesting.