Remdesivir: COVID's Standard of Care that May be Causing more Harm than Good (REVIEW)
Part IV: Examining the Toxicity & Concluding Remarks
So we’ve examined Remdesivir’s therapeutic effects and what factors may play a role in it’s effectiveness.
This leads us to the next topic. We’ve heard of anecdotal evidence of renal and hepatotoxicity coming from COVID medical staff as well as the family members of loved ones treated with Remdesivir.
But we’ll need more than anecdotal evidence, so let’s dive into the literature and see what we can find.
As I said before, the evidence is going to be nuanced and will require a lot of interpretation. Remember that we may not get an absolute answer, but we can still see what the literature has to offer.
Clarifying some Terms
There are three main organs we will be looking at to examine for toxicity and possible organ damage. Those will be the liver, kidneys, and the heart.
In order to measure possible damage specific biomarkers are analyzed. The term biomarker is a portmanteau of “biological markers” and tends to refer to a set of different medical measurements. For those who would like an in-depth dive into the term I suggest this paper.
However, for the sake of simplicity we will go with the idea that biomarkers are measures of structures, compounds, or any measurement that may indicate an interaction between a possible hazardous substance and a biological/organ system. In short, these are measures that would indicate a negative change of state.
Usually damage or altered functionality of an organ changes the presentation or metabolism of certain compounds. Therefore, measuring for changes in the presence of certain enzymes or proteins would point to possible damage.
Luckily, the liver and kidney produce different compounds, which can be measured for possible damage.
Liver
There are several biomarkers than can be measured for the liver.
Aminotransferases: Aminotransferases refer to a group of enzymes that are responsible for converting amino acids between one another through the addition/removal of functional groups. In particular, the most common aminotransferases measured in clinical studies are the alanine and aspartate aminotransferases (shortened to ALT and AST, respectively), which are responsible for the interconversion between the amino acids alanine and aspartate through either the addition or removal of a carboxylic acid group.
Elevated measures of these enzymes may indicate increased enzyme expression, possibly due to the presence of an exogenous substance, or it could be an indicate of hepatic cell damage. Therefore, sudden increases in aminotransferases are considered a good measure of drug-induced hepatotoxicity.
Bilirubin: Bilirubin is a metabolite of hemoglobin and is derived from the catabolism (breakdown) of red blood cells. Red blood cell metabolites are moved to the liver where it is mixed with bile in order to be further metabolized and excreted. Hepatic injury or impaired function may decrease the clearance of bilirubin, and so elevated bilirubin levels may be another indicator of drug-induced hepatotoxicity.
Kidneys
Creatinine Clearance (CrCl): The only test for kidney function we will come across here examines creatinine clearance. Creatinine is a metabolite of the compound creatine. Creatine itself is utilized by muscles as a phosphate transporter and helps provide energy to muscles, which is why it is one of the most commonly used compounds in the fitness industry. Creatinine is cleared through the kidneys via urine, and so reduced measures of creatinine may indicate impaired renal function. Therefore, creatinine concentrations in urine, compared to serum levels of creatinine, may indicate whether the kidneys are able to filter and excrete creatinine adequately.
Heart
Electrocardiogram (ECG or EKG): The case studies and reports on altered heart function here will be based on ECG measures. More specifically, the ECGs are used to measure the rate of heartbeats. Reduced heart rates, and possible evidence of bradychardia (heart rates below 60 bpm) are used as indicators of possible altered heart functionality due to Remdesivir.
So with these in mind we can examine the literature and see if there is evidence to support the anecdotal evidence of Remdesivir’s toxicity.
EVIDENCE IN THE LITERATURE
Kidney and Liver Function
Most papers will group kidney and liver function together so I will do the same here. Keep in mind that late stage symptoms of COVID include kidney and liver damage, and so we’ll have to examine the evidence within that context.
In general, Remdesivir use was correlated with elevated levels of aminotransferases and bilirubin. However, these levels tended to return to normal after stopping the use of Remdesivir. Furthermore, there doesn’t seem to be substantial evidence of liver and kidney damage. Many factors may attribute to impaired kidney and liver function, including COVID and synergistic effects from other drugs.
This is highlighted in a study by Laar et. al. 2021, in which kidney and liver biomarkers were measured in 103 patients. As the results indicate, there seemed to be elevated levels of AST and ALT up to 15 days after Remdesivir administration began, although these returned to baseline, and may be one part of several factors that may lead to possible adverse events. It’s also important to note that the patients included in this study were on supplemental oxygen, yet the researchers did not indicate if ventilation was required.
The researchers noted (emphasis mine):
“The incidence of decreased glomerular filtration rate was comparable with data reported in the RCTs last year. 7 , 8 A quarter of our population had AST elevation and a third ALT elevation, which is more than the adverse events reported in RCTs (3.4–5% and 2.3–6%, respectively). However, Beigel et al. only reported adverse events of grade 2 or higher; Wang et al. and Goldman et al. did not specify the grade of adverse events included in the study. 1 , 7 , 8 Most of the liver transaminase elevation in patients in our study was mild (grade 1). Only 1% showed grade 3 elevation, whereas Goldman et al. reported grade 3 and 4 adverse events in 2–6% of the patients. 8 None of the patients that started with transaminases above ULN, including patients meeting exclusion criteria, had grade 2 or higher transaminase elevation.”
If we look at the liver specifically, we can see that the evidence is conflicting there as well.
Here’s an excerpt from Sodeifian et. al. 2021 discussing possible drug-induced liver toxicity from Remdesivir (emphasis mine):
“The first study reporting the safety of remdesivir for COVID-19 patients, conducted by Grein et al., investigated the effect of 5 to 10-days courses of remdesivir on the changes in the category of oxygen-support status in a small cohort of 53 patients. The most common adverse event in this study was increased hepatic enzymes by an incidence of 23%. Moreover, one of the four patients who discontinued the treatment was due to the elevated liver aminotransferase (20). A similar pattern was replicated in the study on 402 patients, evaluating the optimum time-course for intravenous remdesivir, conducted by Goldman et al. In that grade, 1-2 ALT and AST elevation (7 and 6% respectively) was reported as the most common liver adverse effects (27). Furthermore, in the placebo-controlled double-blinded clinical trial on a total sample of 255 patients, conducted by Wang et al., grade 1–2 Increased AST was detected as an adverse liver effect (12% in the placebo group, 7% in remdesivir group; or 12:7%) and grade 1–2 increased ALT led to drug discontinuation (1%). However, the most common liver adverse effects reported by the same study were grade 1–2 hypoalbuminemia (15:13%) and grade 1–2 increased bilirubin (9:10%) respectively, latter of which also caused drug discontinuation (1%) (23). In an exciting perspective open-labeled study, remdesivir induced adverse effects were compared between patients in an intensive care unit (ICU) and infectious disease wards (IDW). While aminotransferase elevation was almost equal among the two groups (ICU = 44.4%; IDW = 41.2%), bilirubin elevation was more probable in ICU rather than IDW patients, suggesting that the differences in the incidence of different adverse effects among further studies may be due to the different severity states of COVID-19 in the patients (24). In addition to this line of studies, there are also some case reports. In a recent one, an acute increase in ALT was reported after 2 days of remdesivir initiation and was corrected immediately following the stop of remdesivir (32). In two other case reports, hepatic enzyme elevation was detected in patients receiving remdesivir with or without HCQ, who were previously treated with lopinavir/ritonavir (35, 36). In another case report, Carothers et al. have suggested that the use of acetylcysteine can be beneficial in the management of acute liver failure (ALF) induced by remdesivir (38).”
There are many factors that may lead to liver injury, and so it’s difficult to fully elucidate Remdesivir as the only causative agent. However, this review mentions a few things that we have come to take as common knowledge now. In some of these studies patients had severe COVID. At that stage they are far more likely to have liver damage from the disease. Taking Remdesivir at this stage would do no good due to the lack of viral replication, and may lead to more adverse events if the patient is already under hepatic damage. Another comment made here indicates enzyme elevations with dosage of Remdesivir in patients who previously were treated with lopinavir/ritonavir. Ritonavir is a P450 enzyme inhibitor which may serve as a hepatotoxin itself. The combination of the two, even through subsequent dosing, may be likely to cause synergistic hepatic damage.
Strikingly, the reviewers noted a case study where the use of acetylcysteine (NAC) in reducing liver damage caused by Remdesivir. For those who are unaware, NAC was an over-the-counter supplement that was suddenly banned by the FDA. The evidence here points to NAC’s use as possibly aiding with hepatic damage from severe COVID as well as reducing drug-induced hepatotoxicity.
So not only do we see that timing and coadministration of Remdesivir with another therapeutic may play a contributing role in liver damage, we can also see that a compound with decades of use, which may benefit patients who suffer liver damage (from both COVID and Remdesivir), has been banned from public use with no evidence as to why, adding more confusion to the FDA’s decision-making processes.
In a very small cohort study, Zampino et. al. found evidence of elevated enzymes and bilirubin in 5 patients prescribed Remdesivir for compassionate use (emphasis mine):
“Our observation supports previous findings obtained in healthy volunteers (Gilead Sciences, data on file) and COVID-19 patients treated with RDV [4, 5], suggesting this antiviral may cause hepatocellular injury. In our patients, this adverse effect neither progressed to severe liver damage nor induced liver failure, although none had a prior chronic liver disease. Although SARS-CoV-2 infection can cause aminotransferase elevation per se, 4 of our 5 patients had normal or slightly elevated AST/ALT levels at RDV treatment start, suggesting a direct role of RDV in hepatocellular toxicity. Despite the overall low number of patients treated, we observed a clear trend of bilirubin elevation with LPV/r and ALT/AST elevation with RDV. Our observation suggests RDV can be used with close monitoring of liver function tests and with caution in subjects with prior liver disease.”
In one pharmacovigilance analysis Montastruc et. al. looked through a database to find evidence of Remdesivir use and hepatic injury. As the researchers found, there was strong evidence of possible liver damage:
Regardless of the association of COVID-19 infection with liver abnormalities, our study suggests an increased risk of liver impairment with remdesivir compared with other drugs. Although values of aspartate aminotransferase, alanine aminotransferase, and bilirubin elevations were not fully available in VigiBase, we found that hepatic disorders were generally serious (in 3 cases out of 4). In most cases remdesivir was the only suspected drug.
And to balance out the evidence, a meta-analysis from Yiting et. al. indicated lack of evidence that points to Remdesivir’s hepatotoxicity.
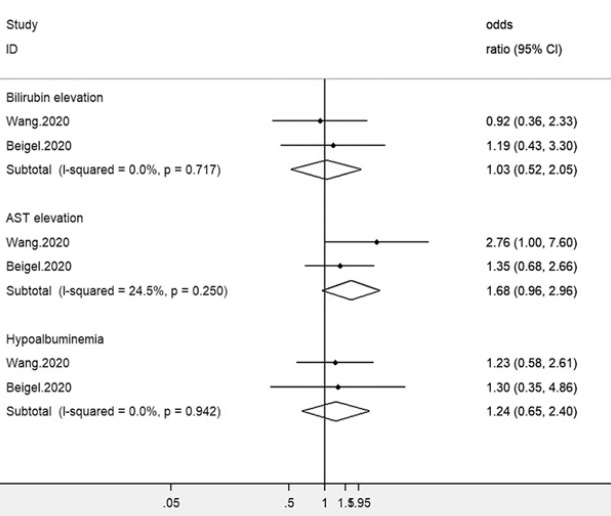
This study likely came from the earliest studies of Remdesivir. We can see the only two studies included were the Wang et. al. study, which was incomplete and indicated elevated enzyme levels. It also included the NIH study, and I’ve already outlined the nuances of that study including the COVID severity/timing of Remdesivir’s role in outcomes. As we can see, this also applies to hepatotoxicity where severely ill patients are more likely to suffer from severe drug-induced liver damage.
When examining the collection of evidence it’s important to examine them all in context. At what stage of the disease is someone in before Remdesivir is administered, is there any evidence of prior liver damage or current damage due to COVID illness, and is there coadministration of a therapeutic that may exacerbate liver damage due to a synergistic effect?
All of these are important factors to take into account when going through the studies. Nonetheless, it seems like there is mounting evidence to indicate a concern with hepatic injury when it comes to Remdesivir.
Next, let’s examine Remdesivir’s possible role in nephrotoxicity.
A review from Fan et. al. indicates inconsistent evidence of Remdesivir and renal injury, although it seems to lean towards possible adverse reaction:
“Although there was no evidence of remdesivir-related nephrotoxicity in Phase I clinical studies, dose-dependent kidney injury and/or reduced function was detected in the repeated dose toxicity studies of remdesivir in animals, which correlated with histopathology findings of renal tubular atrophy, basophilia and casts [3]. Grein et al. reported renal impairments, acute kidney injury and hematuria in 8 %, 6 % and 4 % of the remdesivir recipients, respectively [2]. A COVID-19 patient, who was treated by our team in Wuhan in March 2020, suffered from acute renal failure after using remdesivir. This case was also reported in a RCT in China [6]. Therefore, it is important to monitor kidney function during remdesivir treatment, particularly for those with pre-existing renal impairments or those receiving combination therapies with other nephrotoxins.”
Again, many factors may be responsible for renal injury and studies should be examine within that context.
In a study by Gerard et. al. researchers looked into the VigiBase database to compare reports of acute renal failure (ARF) from Remdesivir against reports from other COVID therapeutics. The study used an odds-ratio calculation. Here, researchers found that ARF was far more likely to occur in patients who were prescribed Remdesivir compared to other therapeutics.
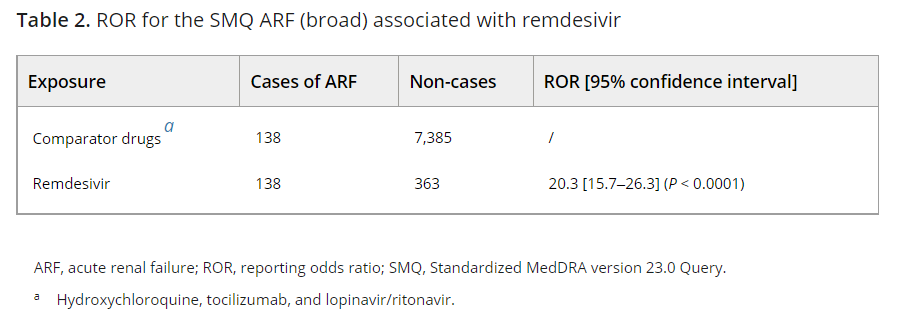
Because this study drew from a database differences in reporting can attribute to the result bias in this study. Also, no demographic data is provided on the patients which leaves one to speculate on the cohorts who suffered ARF, although we can speculate they are older and more likely suffer from comorbidities. Nonetheless, the odds ratio here indicates an extremely disproportionate signal against Remdesivir.
However, another group of researchers (Chouchana et. al.) conducted a more recent review of the VigiBase and looked for evidence of kidney disorder cases and found greater rates of kidney disorders in those who took Remdesivir as compared to other therapeutics. This seemed to occur in all therapeutic instances and even in those that suffered severe or critical COVID.

This would look like concrete evidence that Remdesivir is, in fact, a renal toxin. If we extend the logic further, we should assume that those who already have renal impairment may be far more likely to experience even more damaging effects from Remdesivir.
However, that does not seem to be the case, and in fact a few studies suggest no severe changes to renal function.
In a study by Ackley et. al. researchers compared incidences of acute kidney injury (AKI) between those who showed renal impairment (ceatinine clearance below 30 mL/min) and those with normal renal function (creatinine clearance above 30 mL/min). Surprisingly, researchers indicated no difference between the two groups in presentation of AKI.
“Despite these confounders, there was no significant difference in the frequency of end-of-treatment (EOT) AKI (5% versus 2.3%; P = 0.283) or early discontinuation due to abnormal LFTs (0% versus 3.9%; P = 0.374). However, patients with an eCrCl of <30 ml/min calculated on the first day of administration of remdesivir had a higher 30-day mortality rate than those with an eCrCl of ≥30 ml/min (50% versus 16.2%; P < 0.001). Of the two patients who developed EOT AKI on remdesivir with an eCrCl <30 ml/min, no cases were attributable to remdesivir administration per the treating physician. One patient was deemed to have developed AKI due to hypotension, and the other patient’s nephrologist deemed AKI to be secondary to either tacrolimus toxicity or contrast-induced nephropathy.”
This creates a complex issue. If those with impaired renal function do not appear to show higher levels of acute kidney injury, how do we explain the prior studies suggesting higher rates of acute renal failure and kidney disorders? Most likely, this may be due to differences in measure. In this study, AKI was considered when patient serum creatinine (SCr) levels were 1.5x above the levels measured on day 1 of Remdesivir treatment. It’s difficult to assess whether these measures would be considered adequate measures for kidney injury, especially since creatinine levels may not be good indicators of kidney health.
The heterogeneity among these studies make it difficult to compare them. However, the small sample size of the Ackley et. al. study compared to the reports in the VigiBase medical database would point towards possible nephrotoxicity.
Remdesivir and Cardiovascular Damage?
When looking up information on possible toxic targets, I didn’t expect to come across possible evidence of heart impairment. Although limited, it does seem like there is some evidence that this could occur.
In a literature review from Nabati et. al. reviewers found evidence of possible signs of possible adverse cardiovascular events due to Remdesivir.
The reviewers concluded:
“Some patients with COVID-19 infection on remdesivir may develop sinus bradycardia, hypotension, T-wave abnormalities, AF, and a prolonged QT interval. Also, few cases of cardiac arrest and complete heat block following remdesivir infusion have been reported. It seems remdesivir have some cardiotoxic and proarrhythmic effects that are especially more pronounced in patients with previous cardiovascular diseases. Continuous cardiac rhythm monitoring is recommended in patients undergoing remdesivir treatment. It is especially advisable in individuals with any known cardiac diseases or electrolyte disturbances. Furthermore, we should be more cautious about additive cardiovascular adverse effects when other drug classes are used in addition to remdesivir treatment. It is due to the fact that their combination may increase the risk of potential fatal ventricular arrhythmias or cardiac arrest. The current safety profile of remdesivir is still not completely known. Further prospective clinical trials can assist us to know more about its safety profile and potential adverse cardiovascular effects.”
In a literature review papers are not evaluated on a comparative basis like what you would expect in a meta-analysis. Instead, a literature review looks at prior studies and picks out any evidence of possible adverse event. It also can’t fully determine if the adverse events are attributable to the drug. It’s a review that requires the most skepticism when viewing the data.
Regardless, it does indicate a concerning signal that would require further studies to elucidate.
Fortunately, we do have evidence from a case series of heart abnormalities after Remdesivir administration.
In this study, conducted by Brunetti et. al, researchers collected serial ECG measures from 52 patients who were given Remdesivir. The researchers saw a significant reduction in heart rate, although there was no indication of arrhythmia or bradycardia (heart rate lower than 60 bpm).
However, several other case studies have indicated instances of bradycardia. In two case reports from Abdelmajid et. al, two patients (one 55-year old male and one 54-year old female) presented with bradycardia after beginning treatment with Remdesivir. In particular, the female patient suffered more severe symptoms that progressed to sinus bradycardia and eventually Remdesivir treatment had to end after 3 days. However, in both instances patients were reported to have recovered.
Another case study, reported by Gubitosa et. al, presents another case of sinus bradycardia. However, in this case the evidence is not as clear cut, as the patient had a prior history of disease including left bundle branch block (LBBB) and hypertension. In this instance, Remdesivir was discontinued as well and the patient made a full recovery.
So not only are the liver and kidney possible targets, but so can the heart. Remember that this was the primary reason Hydroxychloroquine had its EUA approval removed. It’s strange that these reports of reduce heart rate and possible bradycardia have not put Remdesivir’s EUA approval in danger. The evidence here may be the weakest, but we need plenty more studies to fully elucidate Remdesivir’s affect on the heart.
WHAT’S THE VERDICT ON REMDESIVIR?
Overall, the evidence that Remdesivir may be toxic to several organ systems is a bit messy. However, there still appears to be a decent number of signals arising from the noise suggesting some concerning symptoms. Of all organs, it seems that the liver may serve as the largest target which is likely typical of a therapeutic. However, evidence around kidney and cardiac function should raise concerns about other organs being a target for toxicity.
For a drug that has been in use for well over a year you would expect more studies than the sparse amount that I was able to collect. The studies I have presented are not conclusive, but they warn of signals that should be looked into further. Considering that Remdesivir is one of the most widely prescribed therapeutics to fight COVID more research needs to be conducted in order to evaluate Remdesivir’s safety profile. As it stands the evidence we do have, although sparse, does suggest something concerning.
But we also have to take into account that many factors are involved with possible toxicity. Underlying health conditions such as liver and kidney disease, as well as cardiovascular disease may contribute to Remdesivir’s possible toxicity. We also know that severe COVID disease can lead to organ damage as well. Considering that this is when Remdesivir tends to be prescribed we can see a possible exacerbation.
Heterogeneity of studies also plays a contributing factor. One study that looks at someone with comorbidities and severe COVID illustrates a different demographic than one that may be young and suffer mild disease. Remdesivir studies utilizing these two groups are likely to produce different results, which may lead to biases in results.
We’ve gone through a lot of data and studies here, so let’s try to summarize everything we have learned about Remdesivir:
Remdesivir has had a spotty record. Prior in vitro studies had remarkable results. However, these did not translate well into clinical trials. The Ebola study showed even worse outcomes with the Remdesivir cohort, although Remdesivir was the only antiviral used in that trial.
The literature is sparse of any studies on Remdesivir and COVID. The main driving force for Remdesivir’s EUA was based on 3 studies; one run by Gilead, one conducted in China which was an incomplete study, and the NIH study (although another study was conducted concurrently). As more studies came out Remdesivir should have been reevaluated for its efficacy. Regardless, the idea that Gilead was able to seek and receive approval on such little evidence should raise many questions.
Remdesivir is likely to see the best benefit when administered as early as possible. This concept doesn’t pertain to just Remdesivir, but all antivirals in general. Treatment at the beginning of a viral infection is less likely to lead to systemic inflammation and thrombotic events. The lower levels of viral particles are also far easier to treat. If doctors are deeply concerned about treating patients it seems absolutely counter-intuitive to wait to administer the drug, especially when patients may suffer complications that could exacerbate adverse reactions from Remdesivir and other therapeutics.
Remdesivir is not effective as a standard of care therapeutic. The most obvious takeaway we can gather from these studies is that Remdesivir, and antivirals in general, should absolutely not be prescribed in those with severe COVID (those who may require ventilation). At that point the therapeutic is likely to have no effect, and we can see that from NIH’s own study which indicated a relationship between disease severity and Remdesivir effectiveness. Patients in this scenario are also far more likely to suffer from organ damage. This would add even more evidence against Remdesivir’s usage here, as it will create an all risk, no reward scenario.
As more evidence arises, we still see no changes being made to Remdesivir’s treatment protocol. Not only was Remdesivir’s treatment regimen a copy/paste from the prior Ebola study, the protocol has hardly seen any change since it has received EUA approval. The only change we have seen was a reduction to a 5-day regimen except for those who require ventilation, in which case this rule makes even less sense because of what we outlined in the second bulletin. This group is the most likely to see harm from Remdesivir’s use since they are already in such a compromised state. Even more ridiculous, now that Gilead has provided evidence of Remdesivir’s effectiveness as an outpatient treatment, why do we not see a move towards outpatient therapeutic? We already have monoclonal antibodies that are provided via IV, and so the lack of adopting Remdesivir in a similar manner makes no sense at all.
There is evidence of some renal, hepatic, and possible cardiovascular damage from Remdesivir. However, multiple factors may affect the prevalence and severity of drug-induced toxicity. All of these should be taken into context when examining studies in order to remove any confounding variables. Nonetheless, some studies have done so and have shown possibly concerning signals. More studies need to be conducted to find further evidence. However, if the idea of “safe and effective” is important we need to question how safe Remdesivir is in the context of how it is being administered.
The medical establishment has bastardized the entire medical process. Remdesivir encapsulates all that we have seen going wrong with the response to COVID. Here, we see a therapeutic with a spotty record, with little evidence behind its safety and effectiveness, being given approval for use at a time where it would see the littlest benefit, that may be considered toxic to many organ systems.
We should seek better from those who are pushing policies that dictate the way we operate our lives. If they couldn’t even be bothered to reexamine their horrendous policies, what does that mean for the rest of us? We have learned so much over the past year, and yet the approach against COVID has not changed aside from the massive push for vaccinations. Whether or not vaccinations are effective, we cannot approach this virus with a one-track mind and expect to see improvement and a healthier, well-off populace.
Remdesivir serves as a reminder that bad policies pervade many of our institutions, and once entrenched will not change, no matter how much evidence comes out to prove otherwise.



