Remdesivir: COVID's Standard of Care that May be Causing more Harm than Good (REVIEW)
Part III-2: Remdesivir in the Age of COVID (Examining the NIH Study)
Now let’s look at the NIH funded study. Note that this is the “Final Report” which means that this is the only study that really matters (I kid, although I’m pretty sure the NIH and FDA are serious)!
This study was most likely used to validate the EUA approval for Remdesivir based on the interim data alone (sound familiar to anyone?). But here we’ll take a look at the complete report.
This study was conducted by Beigel et. al. 2020 and funded by the NIAID (it also goes by the clinical trial name ACTT-1).
In this study researchers examined if Remdesivir could reduce the number of days of hospitalization as a primary outcome.
Immediately we should see something different with this study. Clinical studies tend to use Mortality as a primary assessment of a therapeutic’s effect. But here, we see that the primary outcome is reduction in hospital days. Why would such a discrepancy in primary outcome be used?
It creates a big issue since the results of this study would not be fully comparable to prior studies.
Another thing to note, the median time from symptom onset until randomization was fairly high at around 9 days. Also, nearly 90% of all the patients had severe disease at the time of enrollment (required oxygen or ventilation).
All of these concerns would suggest that Remdesivir may not be as effective as promised.
However, the results indicate greater reduction in duration of hospitalization as well as reduction in mortality (emphasis mine):
Patients who received remdesivir had a shorter time to recovery (the primary end point) than those who received placebo (median, 10 days vs. 15 days; rate ratio for recovery, 1.29 [95% CI, 1.12 to 1.49]) and were more likely to have improvement in the ordinal scale score at day 15 (key secondary end point; odds ratio, 1.5; 95% CI, 1.2 to 1.9). Additional secondary end points supporting these findings include remdesivir treatment resulting in a shorter time to improvement of one and of two ordinal scale categories, a shorter time to discharge or to a sustained National Early Warning Score of 2 or lower, and a shorter length of initial hospital stay (median, 12 days vs. 17 days). All-cause mortality was 11.4% with remdesivir and 15.2% with placebo (hazard ratio, 0.73; 95% CI, 0.52 to 1.03).
There’s a lot to take into consideration here, and we’ll need to attempt to interpret the data.
First off, a comparison needs to be made between reduction in hospitalization and mortality. Even though there seems to be a large difference in recovery time, there still seemed to be a relatively high level of deaths within the Remdesivir group, and the researchers made note of that as well. This creates an issue; who are the ones likely to improve sooner, and who are the ones likely to die?
It seems like we need to lay out a few ideas to take into consideration here:
The game of absolute vs relative risk: This study, like many clinical studies, is a comparison study between a treatment group and control/placebo group. Calculation on that front tend to act as a relative risk reduction (if given a therapeutic how likely is one to get better/survive). However, absolute risk takes into account how likely one is to die/get worse overall.
End-point timing: Two measures of end-point times are used; one at 15 days and one at 28 days. On it’s own this may not seem like an important measure, but these can be used to determine delayed time of recovery or death. Remember that the primary measure here is reduced time of hospitalization, which we can use to assume that those who are in the control group may have delayed recovery time and can be seen within the 15 and 28 day end-point measures.
Baseline Score: Depending on where patient’s starting points are (no oxygen needed, oxygen needed, ventilation, etc.) can determine a lot about the patient’s possible outcome and can be used as a predictor of Remdesivir’s effectiveness.
The best graphs to discern this study is the one posted below:
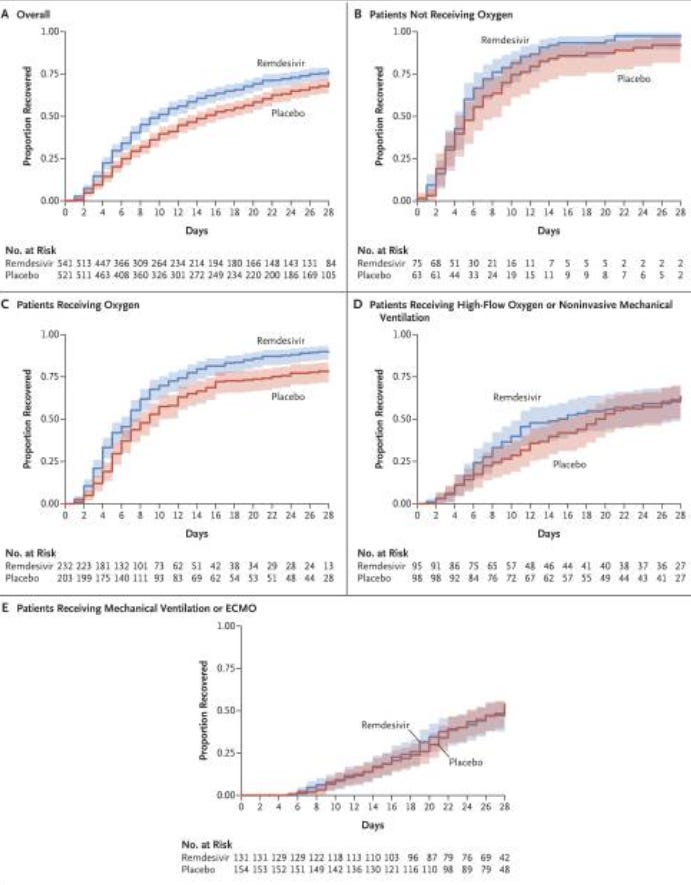
The overall graph gives a good depiction of the difference between absolute and relative risk. Here, we can use a qualitative approach and use the “gap” between the two lines as an odds ratio factor. We can see here that the gap suggests Remdesivir is superior than placebo alone.
However, if we look at the proportion recovered we can see a different story. By the 28-day endpoint around 15% of Remdesivir patients did not show improvement, either meaning they became deceased (deceased always counts as not recovered) or were still ill. However, in the placebo group that number was around 20%. The relative improvement here may be around 75%, and yet the absolute improvement would be around 5%; hardly an endorsement for a drug, as it seems that outcomes regardless of treatment have slightly low odds of improving. This is the same issue we saw with Molnupiravir where the final report indicated around a 30% reduction in hospitalizations/death while the absolute risk was around 3%.
The use of the overall data poses some serious problems. You may have noticed that the group with the largest sample size was the group requiring oxygen. I will talk about this group specifically, but it seems like this group, even as indicated by the researchers themselves, are the most likely to benefit from Remdesivir. We will have to look deeper into that to see why.
One great thing about seeing this stratified data is that we can see how Remdesivir affects different cohorts based on severity of illness. We’ll use some interpretations and inferences here, but we should be able to rationalize why there seems to be a difference among the cohorts.
If we move onto Graph (B) you’ll notice a nearly universal improvement for both the treatment and control group. This group is a little hard to decipher, but let’s consider who is likely to fall into this group. We are looking at people who are hospitalized but do not require oxygen and both groups did not progress into more severe COVID. Also, take into consideration that even after 9 days of symptom onset these patients have not progressed into needing oxygen. Based on this information, we can infer that this group would be likely to have fewer comorbidities, likely to be younger and overall healthier (essentially any factor that is less likely to lead to severe illness). In this group it seems that, irrespective of treatment or control group, these people are far more likely to do well against SARS-COV2 overall, and we can see that with the same 28-day endpoint result.
Compare the percentage of people who did not improve after 28 days in the Remdesivir group (2.7%) to the control group (3.3%) and we hardly see any benefit. However, if we argue that those who are younger are far more likely to not progress into requiring oxygen, how do we explain the youngest demographic showing the greatest improvement when provided Remdesivir, as seen in Figure 3?
A way to rationalize this is to think about shipping. Let’s say you order a package online (it’s Christmas time, we know you’re doing it!) and you have a choice of either 2-day shipping or 3-5 business days. Either way, you are getting your package (going to improve), the issue is how quickly you would want your package. The same may be occurring here, where the group who is not receiving oxygen is likely to improve regardless if they receive treatment or not. Treatment most likely accelerates the improvement in this group (look at the sudden drop between days 4-8), even though they may not need it. These people are also far more likely to be at the time frame where viral replication and spread is still occurring, meaning that Remdesivir will have the greatest effect at this stage of the disease. However, many factors may align to not produce severe illness within these groups and so they are likely to improve regardless if they were provided Remdesivir.
Now, let’s look at Graph (C). I think this is the most important graph, as it serves as a pivotal point in the disease progression, and one that highlights the most important inflection point where antivirals may begin to lose efficacy.
Here, we can see the greatest benefit with the use of Remdesivir. This group contained the largest number of patients and so we can assume this group to be the most powered. But why is it this group that we see such a large discrepancy between the two groups? It could be that this point is where many patients progress into the next phase of the disease where widespread inflammation takes over instead of viral infection.
Treatment with Remdesivir at this stage may prevent a soon-to-be progression into chronic inflammation, at which point there may be a greater likelihood of worse outcome as the treatment options would become far more limited and the disease more difficult to treat.
“Saving” some of the Remdesivir group from progressing into worse disease would explain the wide discrepancy with a relative improvement of nearly half.
Now, I will admit this is all completely speculative, and I may be reading too much into the tea leaves (which is certainly something no one should get deep into). However, there is a pattern laid out in between the cohorts, and there has to be a reason why the results are the way they are.
To add more support to the argument made in support of Graph (C)’s results, we can look at both Graphs (D) and (E) where we can see that the outcomes were the same in both groups. At this point, patients require ventilation in both cohorts. There’s no doubt that at this stage this is predominately a disease of inflammation, and so we can see why the outcomes at the 28-day endpoint are the same; Remdesivir is no longer effective at this point because viral replication is not occurring to a high enough degree to have an effect.
What’s even more interesting is that there still seems to be a noticeable gap that emerges between the two groups, which would add weight to the idea that Remdesivir may reduce recovery time. We can argue that even though Remdesivir is less effective, there may still be patients who may see some benefit. This would also explain why that gap has been mostly diminished in the mechanical ventilation group.
I’ve said a lot here so let’s try to summarize and put it into perspective. Remember that this is intended to be informative, and the information here (and everywhere else) should not be used as justification to be prescribed or to take medications:
Most people will make a fully recovery with COVID. This may be an extremely controversial statement, but we have plenty of evidence to suggest this is a likely outcome for many people who become ill, even if they may not receive treatment. This may explain the results in Graph (B) where even though nearly all patients in both groups made an improvement, the Remdesivir group seemed to have seen greater improvement earlier in the course of the study. Even if this is true, this scenario is a very dangerous one to consider, because most people would be left to speculate whether they are likely to make a full recovery without any treatment options. Especially now that we have made it pretty clear that treatment before more severe symptom onset is best it’s a dangerous game to assume if it means that treatment may be delayed.
There is a noticeable “inflection point” where antivirals may begin to lose effectiveness. The turn into “requiring oxygen” seems to signal a dangerous point in treatment where difficulty breathing may be increasingly caused by inflammation of the lungs and possible fluid build up. In this study Remdesivir saw the most effect here, possibly because it may have prevented the transition into severe, inflammatory disease for several patients. Before this point, Remdesivir will be the most beneficial as viral infection would dominate. However, as patients move towards requiring more support through ventilators Remdesivir and other antivirals will see less efficacy here. From that point onwards the odds of improving will begin to decline.
Late stage disease will most likely not see improvement with antivirals. The final 2 graphs show how patients suffering from chronic inflammation and thrombotic events see no improvement with antivirals. At this late stage of the disease the effects are minimal.
I have taken a lot of liberties with evaluating this study (probably more than I should), but we can see apparent differences in outcomes based on stages of the disease.
It’s strange that the researchers did not dive deeper into the differences in outcomes between cohorts, although they did mention that those with less severe disease (lower ordinance scores) are likely to see the biggest improvement:
However, the interaction tests suggest greater benefit (with respect to recovery and mortality) in lower ordinal score categories. This should not be interpreted as conclusively showing a lack of efficacy in higher ordinal score categories. The median recovery time for patients in category 7 could not be estimated, which suggests that the follow-up time may have been too short to evaluate that subgroup.
The results of this study suggest that Remdesivir may be effective, but from the stratified data we can see clear evidence that the greatest level of benefit will be achieved at lower disease severity. Again, more striking evidence of the importance of early treatment.
This can be confirmed with two extra studies. I’ve gone on too long, so I’ll summarize these.
At the same time the ACCT-1 trial was occurring, the NIH set up another trial to examine Remdesivir use in moderate COVID patients. In this study (Spinner et. al. 2020) Remdesivir was prescribed to moderate COVID patients at either a 5 or 10 day course. The researchers found greater odds of improvement within the 5-day treatment group.
Interestingly, Gilead recently released the interim results of an out-patient study. In this study patients were provided either a 3 day IV course of Remdesivir or placebo (emphasis mine):
In an analysis of 562 participants randomly assigned in a 1:1 ratio to receive Veklury or placebo, Veklury demonstrated a statistically significant 87% reduction in risk for the composite primary endpoint of COVID-19 related hospitalization or all-cause death by Day 28 (0.7% [2/279]) compared with placebo (5.3% [15/283]) p=0.008. Results also showed an 81% reduction in risk for the composite secondary endpoint of medical visits due to COVID-19 or all-cause death by Day 28 for participants treated with Veklury (1.6% [4/246]) compared with placebo (8.3% [21/252]) p=0.002. In the study, no deaths were observed in either arm by Day 28.
These results are even better than both the in-patient studies and the Molnupiravir MOVe-OUT trial, although it seems they may have ended the study short of the emergence of Delta. Nonetheless, we continue to see more and more evidence that early treatment is best, and yet most treatment depends on waiting until a patient is in a hospital before the treatment begins. At that time the chances of treatments being effective are greatly reduced, and we even see that reflected in the NIH study.
So Remdesivir may see the greatest benefit when used early. The question remains though; how toxic is Remdesivir? It seems that we may be witnessing the same issues as Hydroxychloroquine, where the timing of a drug is likely to lead to more adverse events.
In my next newsletter I will dive into literature to see if there is any evidence of toxicity. Note that this will be messy, and we may not be able to get clear evidence. Keep in mind the idea that “the cure can’t be worse than the disease” and that a drug should always be assessed on a risk/benefit analysis that depends on what stage of the disease someone may be in.