Remdesivir: COVID's Standard of Care that May be Causing more Harm than Good (REVIEW)
Part II: The Remdesivir Evidence before the COVID Pandemic
I would prefer to keep my evidence data together. However, my analysis of the COVID-19 Clinical Trials are taking up a lot more space than I anticipated. So I have decided to break up the studies from before the pandemic and those looking at evidence against SARS-COV2 directly.
EVIDENCE PRIOR TO COVID-19 PANDEMIC
Early studies with Remdesivir showed broad spectrum antiviral activity against many viruses, including coronaviruses such as SARS-COV and MERS-COV as well as Ebola.
As taken from Pardo et. al. 2020:
Remdesivir exhibits broad in vitro antiviral action against zoonotic and human pathogens from multiple virus families (Table 1). Remdesivir’s activity has been consistent when tested against members of the Filoviridae, Paramyxoviridae, Pneumoviridae, and Coronaviridae. 6 Among HCoV, remdesivir inhibits three of the endemic strains associated with respiratory illness (HCoV-OC43, 229E, and NL63) as well as the less common MERS-CoV, SARS-CoV, and novel SARS-CoV-2.7–9,24 In addition, remdesivir possesses activity against SARS-like and MERS-like bat coronaviruses (HKU3, WIV1, SHC014, and HKU5). 7 Although most preclinical research has been in vitro, remdesivir was also effective in non-human primate (NHP) models of MERS, Nipah virus infection, and EVD.5,25,26
Nonclinical Studies
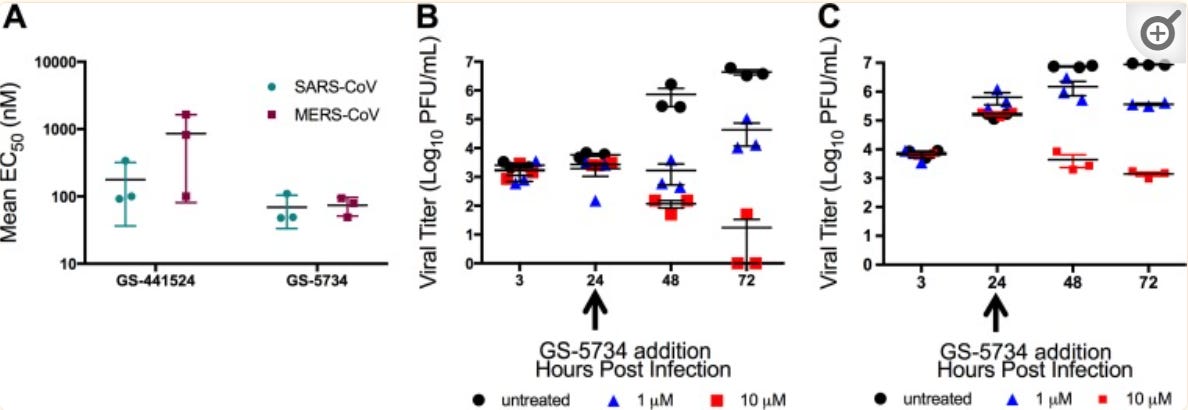
In a study by Agostini et. al. 2018 researchers showed inhibition of several strains of coronavirus, with the results collected and represented by the graphs below.

The researchers also showed inhibition of both SARS-COV and MERS-COV in a dose-dependent manner. It also indicated that administration of Remdesivir 24 hours post-infection still showed a reduction in viral load, indicating possible evidence towards the importance of early treatment. In this study, Primary human airway epithelial (HAE) cells were used as models for lung infection by both SARS-COV and MERS-COV.
When it comes to animal studies, the biggest study on Remdesivir and Ebola occurred in rhesus monkeys. A study by Warren et. al. 2016 looked at inoculated rhesus monkeys and the effects on Ebola biomarkers, survivability, and Ebola viral load.
This study was broken into two parts. In Part 1, rhesus monkeys were inoculated with Ebola and provided either a 12-day vehicle (contains all of the reagents in the serum aside from Remdesivir) or a Remdesivir solution starting on either the day of inoculation or 2 days afterwards. Although the treatment group saw reduced viral load and mortality, the treatment dosage (3 mg/kg body weight) was considered too low.
Part 2 of the study consisted of providing a 10 mg/kg body weight loading dose followed by daily 3 mg/kg body weight starting either on day 2 or day 3 post-inoculation. Another group was provided 10 mg/kg body weight Remdesivir daily 3 days post-inoculation. This group seemed to see the greatest survivability and improvement.
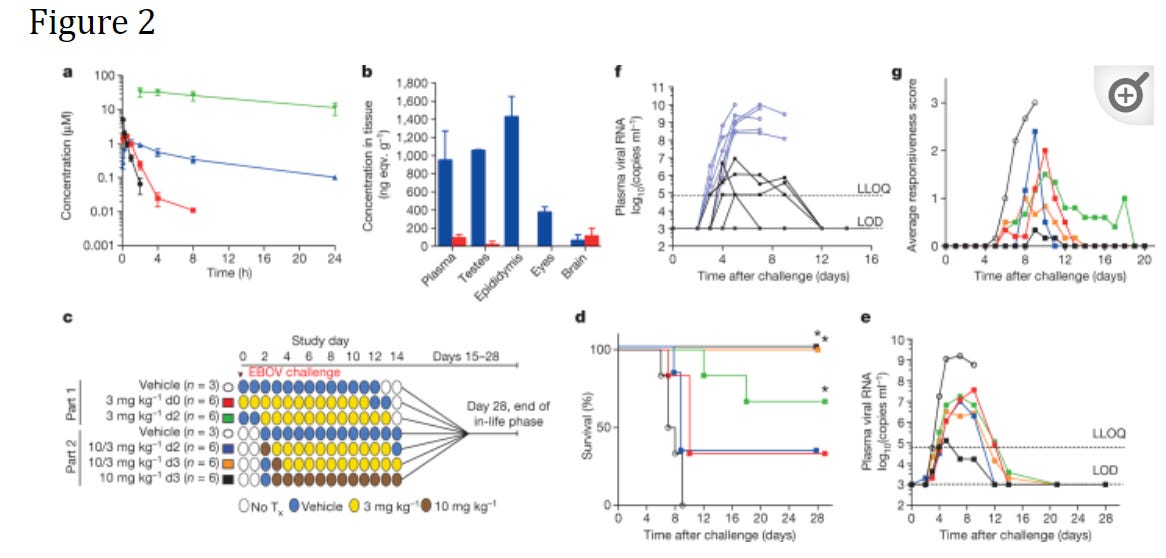
This study was important to both the efficacy and safety of Remdesivir as no prior animal and clinical studies were conducted at the time of this study.
As the authors concluded (Note that NHP refers to non-human primates, EBOV refers to Ebola Virus, and NTP refers to nucleotide triphosphate- Remdesivir’s active metabolite form):
In summary, GS-5734 is a potent and selective inhibitor of EBOV in multiple relevant permissive cell types. In healthy NHPs, intravenous administration of GS-5734 resulted in rapid accumulation and persistence of intracellular NTP. In an NHP model of fatal EVD, pronounced antiviral effects, amelioration of EVD signs, and significant survival benefit was achieved despite treatment initiation on day 3, a time when systemic viral RNA was detectable. These results represent the first case of substantive post-exposure protection against EVD by a small-molecule antiviral compound in NHPs.
With these studies, Remdesivir was considered a possible candidate to fight Ebola, and it was many of these studies that would later support the examination of Remdesivir as a possible candidate against SARS-COV2. However, it’s important to remember that nonclinical studies don’t always translate well into human trials.
Clinical Trial Against Ebola
There haven’t been many clinical trials for Remdesivir prior to COVID. This is most likely due to its recent adoption as an antiviral (it was selected from the database around 2014), but there have been a few case studies and one concerning clinical trial in particular.
As taken from Pardo et. al. 2020:
A limited number of case reports described the use of remdesivir for EVD through emergency compassionate use protocols prior to the completion of formal clinical trials.27,28 The first case described the use of remdesivir for EBOV meningoencephalitis in a 39-year-old woman who had fully recovered from an episode of EVD 9 months earlier. The second case involved an infant diagnosed with EVD on her first day of life following birth from an EBOV-positive mother. 28 Although both patients in these reports survived, it is difficult to make conclusions regarding the role remdesivir played in their recovery as multiple therapies were administered.
Aside from the very limited case studies in which the efficacy of Remdesivir is hard to discern due to other therapeutics, only one RCT involving Remdesivir and Ebola tends to appear in the literature.
In a study conducted by Mulangu et. al. 2019 researchers examined the use of Remdesivir and 3 types of monoclonal antibody treatments against Ebola. The study was conducted using patients from the Democratic Republic of Congo. The therapies involved in the study include Remdesivir, a triple monoclonal antibody treatment called Zmapp (standard of care for Ebola and used as a reference group), a single monoclonal antibody called MAb114, and another triple monoclonal antibody treatment named REGN-EB3.
Patients were included based on positive PCR Ct counts and were randomized to 1 of 4 treatment groups.
As can be seen, Remdesivir showed the worst outcome in mortality as compared to the Mab therapeutics.

Taken from Mulangu et. al. 2019 (emphasis mine):
The percentage of patients who died was lower in the MAb114 group and in the REGN-EB3 group than in the ZMapp group (Figure 1 and Table 2). The difference between the MAb114 and the ZMapp groups was −14.6 percentage points (95% confidence interval [CI], −25.2 to −1.7; P=0.007); the difference between the REGN-EB3 group and the ZMapp subgroup was −17.8 percentage points (95% CI, −28.9 to −2.9; P=0.002); and the difference between the remdesivir and ZMapp groups was 3.4 percentage points (95% CI, −7.2 to 14.0). (Fig. S5 shows the differences in mortality in the remdesivir, MAb114, and REGN-EB3 groups relative to the ZMapp group according to Ct value, age, sex, and site.) The survival benefits seen in the MAb114 and REGN-EB3 groups were also seen in sensitivity analyses adjusted for potential baseline imbalances (Table 3 and Table 4 and Table S3).
Using the ZMapp group as a reference group, negative percentage points indicate lower mortality in both the REGN-EB3 and MAb114 group while Remdesivir showed a higher mortality rate (positive percentage points).
The researchers also examined a secondary efficacy end point where viral clearance was achieved in living patients. A deceased patient would be considered to have not had viral clearance. On this part, Remdesivir showed a clearance day of greater than 28 days due to the level of mortality that occurred within that treatment group.
The results of this clinical trial would indicate damning evidence against Remdesivir, but it’s important to keep the information in context. Remdesivir was compared to a standard of care therapeutic and not a placebo, meaning that its efficacy provides a relative value. This doesn’t mean that Remdesivir is ineffective, but may suggest that it is less effective than what was normally used as standard of care. It’s important to make this distinction when examining clinical trials and understanding if a comparison is made between another treatment or no treatment (placebo).
Also, the distinction between SARS-COV2 and Ebola makes it hard to argue a translational effect. A drug’s effect on one pathogen cannot be used as a measure of its effectiveness against another, especially when the pathology of the pathogens are very different. As can be noted, Ebola is an extremely pervasive virus that can be found to circulate in many regions of a person’s body. Also, those who have been infected with Ebola also may have prolonged viral RNA presence which may lead to a reemergence of symptoms or possible infection of others (which explains the bar graph provided by Warren et. al. 2016).
However, even in this study the authors made note of the importance of timing and dosage when it comes to clinical outcomes (emphasis mine):
In addition to differential effects of the four trial agents with respect to mortality, the results showed the importance of early diagnosis and treatment. We observed an 11% increase in the odds of death for each day that symptoms persisted before enrollment. These data highlight the need for community awareness that earlier diagnosis and treatment are associated with increased survival…
Given that 97% of deaths in this trial occurred within 10 days after enrollment, the efficacy of MAb114 and REGN-EB3 as compared with that of ZMapp and remdesivir might be partly attributable to the fact that the full treatment courses of MAb114 and REGN-EB3 were administered in a single dose, whereas ZMapp and remdesivir were administered in multiple infusions.
So whether or not the result of this study could be used as a testament to Remdesivir’s efficacy (or lack thereof), we still see a consistent argument in favor of early treatment irrespective of the disease.
Nonetheless, the accumulation of prior evidence of Remdesivir against many RNA viruses still provides support to further look into Remdesivir as a possible drug candidate against COVID.
In my next newsletter, I will go over the studies that examined the effect of Remdesivir on SARS-COV2. Make sure to brace yourself, it’s a lot to go through and there may be some sketchy things going on.
Thank you for reading my newsletter. If you enjoy my articles please consider becoming a free subscriber in order to receive notifications.
And share with others who may find these newsletters interesting.
Also, please consider becoming a paid member. The research and work put into these articles takes many hours and being a paid subscriber allows me to continue to do this full time.



