Remdesivir: COVID's Standard of Care that May be Causing more Harm than Good (REVIEW)
Part I: Introduction, Brief History and Mechanism of Action
Last week a Twitter post began circulating in which a nurse talked about standard of care drugs doing nothing to help with COVID. In particular she mentions Remdesivir. Not only does she talk about it not being effective, but she mentioned several instances of organ failure occurring in some of the COVID patients.

I remember liking and retweeting the post, but not because I fully believed the claims to be true; it was because I have seen this comment made several times before.
A few weeks ago another Twitter post was made from a young man describing his mother’s passing due to her COVID treatment which included Remdesivir. I can’t find the post now so maybe Twitter removed it, but I remember him mentioning kidney failure as the possible cause of death.
And even within my own Substack community I saw someone post a comment about a loved one who was being hospitalized for COVID and being given Remdesivir, much to that person’s dismay.
I never put any weight into the possible toxicities, mainly because Remdesivir just seemed like an ineffective drug against SARS-COV2 sans adverse side effects.
But recently a family friend was diagnosed with a deadly disease, and hearing some of the absurd questions raised by the caring physicians made me think about the practice of medicine and how versed many doctors are when it comes to the science and evidence. This isn’t something I planned for, but the ethics of medicine is something that I now can’t get out of my head with everything going on with COVID.
So I wanted to take a look and see what I can find on Remdesivir and really see if there is any validity to the concerns around Remdesivir’s toxicity. The layout of this piece will be similar to all of my other posts; I’ll describe Remdesivir and its mechanism of action and brief history, look at both nonclinical and clinical evidence, and finish with looking at whether there is any concerns in regards to hepatotoxicity (liver toxicity) and renal toxicity (kidney toxicity).
Before going in, a warning that these posts will not provide a direct answer (just like all posts of this nature), and instead will provide nuance and perspective to draw from.
Remdesivir’s History and Mechanism of Action
Brief History
Remdesivir may have one of the most recent histories when it comes to COVID therapeutics.

As taken from this review by Eastman et. al. 2020, Remdesivir was part of a group of nuceloside analogues developed to fight against many global pathogens:
Remdesivir (GS-5734) was developed by Gilead Sciences and emerged from a collaboration between Gilead, the U.S. Centers for Disease Control and Prevention (CDC) and the U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID). They sought to identify therapeutic agents for treating RNA-based viruses that maintained global pandemic potential, such as those that indeed emerged following the initiation of the program, including EBOV and the Coronaviridae family viruses exemplified by Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS).
It wasn’t until 2014 with the Ebola outbreak that this database was utilized to find a possible antiviral agent to fight Ebola.
When the Ebola outbreak occurred in 2014, the assembled library was utilized to identify and prioritize compounds with efficacy against EBOV. The study by Madelain et al. found that GS-5734 reduced EBOV replication in HeLa cells with an IC50 ≈ 100 nM, and it retained potency in in vivo nonhuman primate EBOV infection models, while GS-441524 was inactive.46,47 In addition to demonstrating activity against EBOV, Warren et al. showed that remdesivir also had antiviral activity against several other viruses, including the coronavirus MERS, with an IC50 of 340 nM in vitro.
So there was evidence, at least in vitro, that Remdesivir is effective against both Ebola and coronaviruses. More shockingly, the IC50 (the drug concentration where 50% of viral inhibition is achieved) from these studies suggested that Remdesivir elicits antiviral properties at very small concentrations. This would mean that low dosage of Remdesivir in human models may achieve antiviral effects, something that tends to be difficult to achieve without causing possible cytotoxicity in clinical trials.
Now, I won’t get into some of the clinical trials with respect to Remdesivir and Ebola until further on, but the evidence from these in vitro studies, in particular those against coronaviruses, led to Remdesivir’s possible use against SARS-COV2.
Mechanism of Action
Remdesivir is a nucleoside analogue of the nucleoside Adenosine with one of the only changes made to the 1’ position of the sugar ring. The only functional difference between Remdesivir and Adenosine is the presence of a nitrile (carbon triple bonded to a nitrogen) at the 1’ sugar position. With naming, the nitrogenous base gets first dibs at numbering. After that, the carbons of the sugar ring get second priority so they use the ‘ notation after the carbon number. The carbon bonded to the base is numbered 1’, then you go around the carbons of the sugar ring ended with the 5’ carbon whose hydroxyl (-OH) group gets phosphates added to it.

Another important piece of information, and one that I have forgotten to mention with Molnupiravir, is that most nucleoside analogues are administered as a “prodrug”, which is the drug before it gets metabolized into its active form (AKA the “active metabolite”).
Nearly all nucleoside analogues are administered as a prodrug, and the modification is always at the 5’-OH group. Nucleoside analogues are never administered as their triphosphate (three phosphate groups) form because the phosphates tend to be negatively charged at physiological pH (physiological pH refers to the body’s typical pH, which is slightly above 7). The ionic charge prevents the drug from passing through cell membranes which tend to be hydrophobic (“water fearing”) in nature and are not permeable to charged molecules. It also prevents the drug from being metabolized by ribonucleases before it can be utilized.
Usually, an additional group is added that provides hydrophobicity to the analogue. What makes Remdesivir interesting is that the prodrug group is a phosphate with additional groups added onto it to remove the ionic phosphate charge. In the case of Remdesivir, this prodrug group is a unique group called “a McGuigan protide”, named after its discoverer Chris McGuigan in 1990.
When the prodrug groups are metabolized i.e. cleaved off of Remdesivir, the drug becomes a monophosphate (one phosphate group attached to the 5’ carbon) and additional phosphate groups are added. Although the evidence is controversial, it seems that the first phosphorylation step (addition of a phosphate group) that turns nucleosides into mononucleotides may be a rate-limiting step (the slowest step in the reaction pathway from a nucleoside to forming a trinucleotide). If this is an issue, bypassing the first phosphorylation step would increase the bioavailability of the trinucleotide form of the drug and thus the antiviral effects would be elicited sooner. There still isn’t enough evidence to support the initial phosphorylation step hypothesis, but having a drug that can bypass this first step may help to improve how quickly a drug acts as an antiviral agent.
Comparatively speaking, the only difference between Remdesivir’s active form and Adenosine is the 1’ nitrile group (the C triple bonded N will either be referred to as a “cyano” group or a “nitrile” group in literature).
Usually, you can confer the type of activity of a nucleoside analogue based on where modifications are made to the original structure. With Molnupiravir, the modification of the nitrogenous base through an additional -OH group engages in base pairing trickery to add incorrect bases during replication. The same effects can be seen with Ribavirin, a guanosine nucleoside analogue that operates as a mutagen, and is utilized as a treatment option for Hepatitis C, where a ring of the nitrogenous base is opened up which alters the base pairing functionality of Ribavirin.
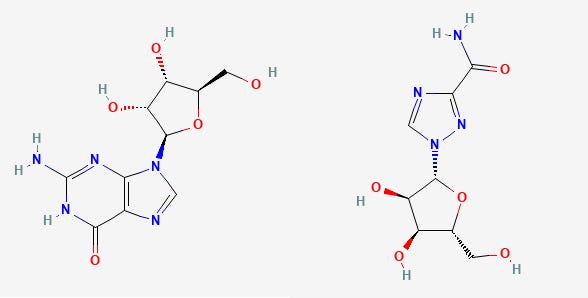
When the sugar ring (the 5-member ring with an oxygen located inside) is modified, usually the intent is to halt the replication process through termination of the elongating strand of the viral genome. Most nucleoside analogues contain modifications at the 3’-OH, which is vital to the elongation step of both transcription and replication since replication occurs in the 5’ → 3’ direction. A modification here is intended to halt the incorporation of other bases, elongation stops, and the replication process is terminated.
Because Remdesivir is a sugar modification, it can be insinuated that it halts the replication process after the virus’ polymerase mistakes it for Adenosine and it becomes incorporated into the elongating strand of SARS-COV2. However, Remdesivir does not directly halt an incoming base. Instead, up to 3 additional bases are added to the elongation strand before no more bases are added and the entire process is stalled.
For a better visual, these images were taken from Gilead’s website about VEKLURY (Remdesivir). You can also find a small video on Gilead’s website with 3D models.

This passive mechanism of action is unique to Remdesivir, and it seems that the nitrile group may engage in electrostatic interactions with some of the amino acids of the viral polymerase.
As taken from the Zhang et. al. 2021 abstract (unfortunately I don’t have access to the full paper, and there’s no way I’m paying the US equivalent of 42 British pounds- which I assume is around 70 bucks):
We found that when Remdesivir locates at an upstream site in RdRp, the 1′-cyano group experiences electrostatic interactions with a salt bridge (Asp865–Lys593), which subsequently halts translocation.
As elongation occurs the RdRp moves the growing strand along. It seems that the nitrile/cyano groups serves as a hook, and as the growing strand moves along the RNA-dependent RNA Polymerase (RdRp) the nitrile group latches onto this salt bridge and halts the strand from moving along. This stall is likely what causes the end of the replication process, leaving an unfinished viral genome. Note that this doesn’t fit with the video on Gilead’s website since that video is most likely not based on any specific mechanism of action.
The ability for Remdesivir to inhibit the replication process of many different viruses highlights the conserved structure of the RdRp across many different viral strains, as it seems that the salt bridge and amino acid interactions detailed above play key roles in interacting with Remdesivir’s nitrile group.
Both Remdesivir’s structure and function make it unique compared to other nucleoside analogues. It indicates that even minor changes to molecules can alter their functionality and take something from being a vital source of both cellular energy (as ATP) or serve as a genetic building block to becoming an agent against pandeming producing virus such as SARS-COV2.
Tomorrow I will post both the nonclinical and clinical studies on Molnupiravir, so please be on the lookout for that post!
Thank you for reading my newsletter. If you enjoy my articles please consider becoming a free subscriber in order to receive notifications.
And share with others who may find these newsletters interesting.
Also, please consider becoming a paid member. The research and work put into these articles takes many hours and being a paid subscriber allows me to continue to do this full time.



The way Remdesivir is currently used makes no sense at all to me. By the time a patient is sick enough to be hospitalized, the viral replication phase of COVID is over already. Hospitals love to administer it though because they get a nice cut of ~$3000 cost per course.