Preliminary Examination of BioNTech Data- Lipid Nanoparticles
Taking a look at some of the data on LNPs, and why this does not bode well for the release of information.
Thank you to the Naked Emperor for sourcing this information. S/he is also analyzing the data (has actually done so before I have) so please take a look at his/her Substack. Many Substacks will be going through the data and so I encourage people to look around to other Substacks as there may be a lot of analyses being done of the information. Also, for those who want to see where all the documents are being stored here is a link.
Update: I was alerted that BioNTech did in fact use the two commercial LNPs below in their formulation (ALC-0315 and ALC-0159). This would provide for something to look into further to see if any literature includes cytotoxicity or possible immune dysfunction. When viewing the information below keep this in mind, and also remember that we are viewing the information retroactively.
Additional update 3/3/2022: I have made another post with several corrections made to this post. Please refer to that post for more accurate information. I apologize that I did not examine these studies further. Also, thank you to Brian from Unglossed for bringing a few of these corrections to my attention. Please check out his Substack.
Right after graduating college I was figuring out what I wanted to do as a career. I know, typical of a millennial to not know what to do after graduation right?
Anyways, I ended up receiving an informal interview with someone at the FDA for an internship. The internship would have entailed examining transcripts and reports sent to the FDA by pharmaceutical companies for whatever new product they wanted to have reviewed.
Now, I did not end up taking the job but during my interview I was shown a few sample papers to give me a gist as to what I would have expected if I took the position.
To my surprise, many of these papers were full of boxes of redactions, to the point where many pages were entirely covered up. I remember the interviewee commenting on one paper and why it had so many redactions (nearly the entire report was redacted), so even though I never took the job I still received my first glimpse into the bureaucratic processes of FDA approval.
We tend to hear of redactions when it comes to the release of government documents with sensitive information, possibly about interactions with other global governments or any activities the FBI or CIA are involved with that many of us are not privy to.
However, we tend not to think of the reports sent to the FDA to examine whether or not to approve of a drug or some item on the market. Remember that the FDA has to approve of pharmaceuticals as well as e-cigarettes.
In many instances personal information about the researchers are removed which is not a surprise. However, what’s surprising is that information in regards to compounds, methodology, and results are likely to be redacted as well, and sometimes to the point that you can’t examine the data at all and give a discerning opinion.
We’ve heard that when the initial data on the Pfizer vaccine would be released to the public via a FOIA (Freedom of Information ACT) request, it would only be released with about 50 pages per month, possibly taking up to 70 years until the full release of the data.
However, a judge ordered a more expedited release of the documents, and just last night the first rollout of the thousands of pages began.
I want to point that this was only brought to my attention by viewing the Substack of the Naked Emperor, so once again please check out this Substack as more information will be uncovered over there to a greater degree than possibly here.
With this release of information I decided to examine one paper more closely, and it’s one paper that examined 3 lipid nanoparticle formulations for possible use in the COVID vaccine.
Lipid nanoparticles are still an entity of ambiguity. A lot of people have considered it a possible agent for the adverse reactions, although they have been in use for quite some time as a drug delivery vehicle, meaning that concerns may be more attributed to the mRNA and the protein production instead as the LNPs are likely to serve as a delivery vehicle.
As the name implies, lipid nanoparticles are molecules of lipids designed to mimic the structure of the phospholipid bilayers that surround all of our cells. But unlike our cells, which contain a negatively charged phosphate backbone, the backbone of lipid nanoparticles are comprised of an amine. The amine can either be quartenary (have 4 things bonded to it) or be tertiary (3 things bonded to it). Either way, the intent of the amine backbone is to provide a positively charged backbone to act counter to our negatively charged backbone.
Like with many things opposites attract, and the intent of these positively charged LNPs is to attract them to our negatively charged bilayers and find entry into our own cells in a manner slightly mimicking that of viruses. Fusion of LNPs to our membranes allows for the release of mRNA into our cells which would then be translated and produce the spike protein.
Therefore, the sourcing of a proper LNP would be considered pretty appropriate.
The paper we will be taking a look at is titled the following:
“EXPRESSION OF LUCIFERASE-ENCODING MODRNA AFTER I.M. APPLICATION OF GMPREADY ACUITAS LIPID NANOPARTICLE FORMULATION”
We’ll start with this introduction into the study:
GMP stands for Good Manufacturing Practice, and it refers to the practice of ensuring that manufactured products and drugs are up to quality standards. This may come about a lot but it’s not a big concern. It just means that the product has been assessed using quality standards, and it usually refers to compounds readily available. Here, the GMP formulation refers to the use of LNPs already on the market (the ALC-0315 and ALC-0159).
The important point of this study is that 3 different LNP combinations were assessed. Only one has available data while two are proprietary (one from the company Acuitas and one from BioNTech’s own in-house formulation).
There’s already a big issue here. We don’t even know the structure used in the other LNP formulations so there’s no way of assessing the structural differences. We can infer from the information above that the LNP used in BioNTech’s formulation is also a PEG-lipid that is 8 polyethylene groups long, but aside from that there is not much help. Therefore, our understanding of these different formulations are already stunted.
I will provide structural information on the available LNPS ALC-0315 and ALC-0159 below:

And for posterity’s sake, here’s the basic structure of a phospholipid to compare:
In short, it appears that the study is broken up into two parts: biodistribution and immune response.
The biodistribution of the LNPs is being measured by the use of a luciferase gene. Luciferase is an enzyme found in fireflies that provides it the glow through an enzymatic reaction, and so the inclusion of luciferase acts as a way of tracking where the LNPs go by checking for “glowing” in mice.
The immune response is comprised of a few factors. It looks like they wanted to see if the LNPs themselves would elicit an immune response to target them, and if the production of luciferase, which would be considered an exogenous (foreign) substance may cause an immune response as well.
Now, the next part is the methodology:
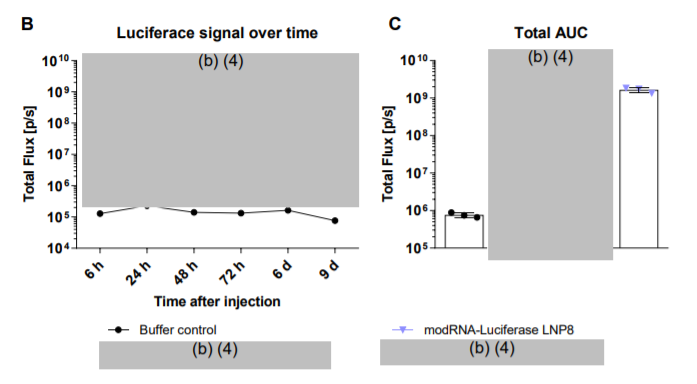
In order to test for luciferase luceferin was provided to the mice which would be enzymatically cleaved and glow. We can see that the timeframes for that are some what reasonable (6, 24, 48, 72 h then 6 d and finally 9 d). However, hardly any measures of immune response were taken. The measures taken 1 day before injection provide for a baseline, yet as we can see only two timepoints were measured for immune response 6 hours and 9 days after.
This large gap in time was apparently due to the isolation of splenocytes to measure T-cell responses by interferons which would indicate an adaptive immune response has occurred, and likely explains the large gap of data. However, that unfortunately means that there would be a large gap in adaptive immune response data overall which may be an issue. Unfortunately, I am not knowledgeable on the way these assays are typically done so keep in mind this may be standard practice.
So here’s the results of the measures for luciferase, and we can clearly see why this is confusing. What we can tell is that two of the LNP formulations saw a nearly 20-fold higher luciferase expression, meaning that most of the LNP uptake occurred around the injection site. For those concerned about travelling vaccines, this is a good sign. The only problem is that we can’t tell which formulation it was!
Yes, we can tell that the GMP-formulated one was part of the pair, but can you tell whether BionNTech’s formulation also reached a 20-fold high expression or could it be the one from the company Acuitas? It also doesn’t help that the use of “LNP8” is used interchangeably, so I can’t tell which one of the formulations it refers to.
The confusion doesn’t end there. Here are the results for the immune response assays:
So we know that two of the formulations led to an innate immune response, although they hint that the one with LNP8 did not elicit as big of an innate immune response. Maybe it means that both the BioNTech formulation and the GMP formulation do not elicit an innate response, although I can’t tell (maybe you guys can!). The important thing to note from this result is that the type of LNP matters when considering eliciting an immune response.
What’s interesting is the last paragraph, which suggests that no adaptive immune response occurred to target luciferase, although it does appear that an adaptive immune response was formed against the modRNA, and it appears that it depends on the type of LNP used.
So for those who are confused as to the reason they performed this study here are the takeaways:
Biodistribution of the LNPs is important. The luciferase measures were done to examine where the LNPs (and by extension the modRNAs that encoded for the luciferase) ended up. The liver was also examined as it tends to be the reservoir for exogenous substances to be metabolized. Finding an LNP formulation that keeps most of the concentration within the injection site is important. Therefore, a balance of high luciferase expression at the injection site and low expression in the liver would suggest that the LNPs did not circulate to a great extent.
The measure of innate and adaptive immune response is to see whether any of the substances being injected would cause their own immune response. That includes the LNPs, the modRNA, and the protein (luciferase). Surprisingly the LNPs elicited their own immune response and it appears that the structure plays an important role (does not help that we can’t examine the differences). Strangely enough the modRNA elicited their own response while the luciferase did not.
Taken altogether, you want an LNP that stays within the injection site and does not travel, does not produce its own immune response, and allows for the release and expression of the intended protein.
One caveat to this is that this analysis was done with mostly the Summary. As I look deeper into the results I may be able to discern some of the information, but as of now it is very difficult due to all of the redactions. Because of this it’s hard to asses how much LNPs actually travelled, and even more important which formulation BioNTech decided to use in the vaccines.
This brings me to my last point.
All Redaction, no Transparency
It’s understandable why some pharmaceutical companies would want to keep certain things proprietary. You may not want trade secrets to get out possibly costing your company millions upon millions of dollar. From a business sense, it’s slightly understandable.
However, this comes at a cost of transparency. Whether due to keeping certain formulations secret or results private, it essentially means that, even with all of this data being released, there’s no way of knowing what was actually found in these studies.
I chose to look mostly at the summary of this study because the results have been mostly redacted. Take a look at this example:
Now, I’m a visual learner and I always turn to figures to help me figure out how to interpret the data. Unfortunately, the redacted figures are even worse:
And see if you can figure out the conclusion of this study:
As a pharmaceutical company, this type of redact is expected. As a layperson, this means that I have no way of knowing what any of this redacted information found or what it means.
Even more important is to keep in mind that the FDA does not receive the full documents, but the redacted ones seen here.
Yes, that means that the FDA’s approval process for many drugs and compounds relies on the FDA not even being able to review the data!
As more and more information is released, documents like these are to be expected, meaning that even after thousands of pages are released we still may not be able to discern any of the information.
This is a type of practice that not only extends to these mRNA vaccines, but to the FDA’s review process as a whole. How could it be that an institution in charge of reviewing and approving drugs cannot even see the data that they are meant to review? What decisions are made to decide on approval? Does the FDA rely solely on the trust of those within the pharmaceutical industry to “do the right thing” when it comes to the products that they produce?
This unfortunately does not bode well for the rest of the data. As many of us here on Substack try to sift through the information many papers may not be redacted, but even still this lack of transparency loses the trust of many people who were already highly skeptical of these federal institutions already.
Even with all that said, I’ll end this post with a white pill. Many people out there have never glanced through the window and have seen how the FDA and bureaucrats operate. With this release of information, and all of its redactions, I am hopeful that many in the public will come to see the level of institutional capture at play. Maybe that’s one form of transparency that these documents can provide us.
And remember, this is not happening to just vaccines but all things that must go through the FDA.
So whether you can make sense of that study, keep this in mind and remember to always be skeptical to what is being presented to you.












"What’s interesting is the last paragraph, which suggests that no adaptive immune response occurred to target luciferase, although it does appear that an adaptive immune response was formed against the modRNA, and it appears that it depends on the type of LNP used."
You may have already spotted it, but 4.5.9 suggests the T Cell / ELISpot response was elicited with the luciferase peptides. A quick review of the research suggests that ELISpot with luciferase peptides is typically used for testing gene vector platforms that are intended to NOT induce an adaptive immune response - https://www.nature.com/articles/3300951 - so, just like here, it may be a common thing that antibody screening usually misses luciferase sensitization and that is why ELISpot is preferred. (Just guessing since I can't find any specific mention of antibodies in conjunction with luciferase gene platform trials.)
So, it probably didn't add a ton of value to the distribution study. A separate immunogenicity study would still be wanted.
There's a non-redacted summary of R-20-0072 and more good stuff in the Australia nonclinical overview that was released last week-ish (including more "spike in the nucleus?" stains) https://www.tga.gov.au/sites/default/files/foi-2389-06.pdf
Lists the redacted products on p 40
mRNA Luciferase LNP12 - DODMA:Chol: DOPE:PEGcerC16 (40:48:10:2); RNA-EH190611-01c, FSU- 1#029, 79% encapsulation, 0.9053 mg/ ml encapsulated RNA, diameter 84 .nm. polydispersity 0.202, storage temperature +4°C.
mRNA Luciferase LNP5 - Acuitas proprietary; RNA-EHl90611-0lc, batch FM-1055-D, 79% encapsulation, 0.924 mg/ ml encapsulated RNA, diameter 108 nm. polydispersity 0.091, storage temperature -80°C.
The lack of transparency in data submitted to federal agencies happens at the EPA also. For pesticide approvals, only the active ingredient in the pesticide has to be declared. The active ingredient can be as little as 1% of the whole formula.
There is an urban myth that the government tests everything and nothing unsafe would ever be released on to the market. We all need to dispel that myth. Talk to family and friends and explain why they can't trust any product. Buyer beware!