Pfizer’s Oral COVID-19 Drug may Interact Similarly to Ivermectin
However, evidence suggests they both may not be effective against SARS-COV2's Protease
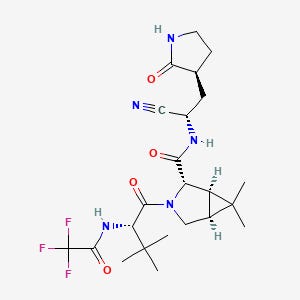
Pfizer’s new SARS-COV2 drug named PF-07321332 has been compared to Ivermectin and has caused concern about a generic therapeutic having slight modifications in order to become patented.
Here I will do a quick comparison of the 2 drugs, their mechanism of action, and how they compare with their structure and activity. This will not determine which one is better but if they may act in a similar manner.
PF-07321332 is being deployed as a 3C-like protease (3CLPRO) so we will compare it to Ivermectin’s protease inhibitory actions.
First off, protease inhibitors are a class of therapeutics that are intended to inhibit the function of proteases. Proteases are enzymes that take newly translated viral proteins and cuts them up into their functional units. Therefore, inhibiting proteases means that nonfunctioning proteins will be produced and lead to nonfunctional viruses.
Protease inhibitors are most commonly found as a cocktail therapy to fight HIV and is usually deployed with a P450 enzyme inhibitor such as ritonavir to prevent the metabolism of the inhibitor. This allows the blood concentration of the protease inhibitor to increase.
The structure of protease inhibitors vary greatly mainly due to their mechanism of action. Protease inhibitors depend upon electrostatic interactions such as dipole-dipole interactions and hydrogen bonding in order to work, and because these interactions may not be fairly strong most protease inhibitors contain a bunch of functional groups to increase the amount of these drug/enzyme electrostatic interactions.
We can see that when comparing the structures of these two drugs:
Note that their structures are very different so we aren’t dealing with a “slight modification for patent purposes” issue with Pfizer’s drug. Instead we can focus on if they work in a similar manner and make a case from there.
The target enzyme here is called SARS-COV2’s main protease 3-chymotrypsin-like protease (3CLpro) but we will just refer to it as “protease” or “SARS-COV2’s protease”.
The protease’s active site, the region of the enzyme that is actively involved in chopping up the enzyme has been elucidated by Ferreira et. al, 2020:

And for those of you who are interested in biochemistry here is how the authors explain the active site’s functionality:
The first step in the catalytic reaction of 3CLpro is the deprotonation of the thiol side chain of Cys145 by His41 for its nucleophilic attack on the carbonyl carbon of glutamine of the polyprotein backbone. Upon its deprotonation, the thiolate ion of Cys145 attacks the peptide carbonyl carbon and forms a thiohemiketal that collapses into the thioester after the cleavage of the peptide bond and release of the C-terminal part of the polypeptide substrate13,19,21. Finally, a water molecule facilitates the hydrolysis of the thioester linkage, displacing Cys145 and releasing the N-terminal segment of the polypeptide substrate. The thioester linkage formation is an essential step in the catalytic mechanism of 3CLpro, and it is targeted in the development of antivirals18.
If that’s too much biochemistry jargon that’s fine. What’s important to note from that excerpt is that a good SARS-COV2 protease inhibitor should interact with the His41 or/and Cys145 amino acid residues of SARS-COV2’s protease.
And in fact we do see that occurring with Pfizer’s drug as noted by modeling done by Pavan et. al, 2021:

And it does seem to occur with Ivermectin as well, as indicated by modeling done by Mody et. al, 2021:

So does this mean that Pfizer’s drug and Ivermectin should work the same? Well yes, but actually no. Even though they both target the active site of SARS-COV2’s protease most protease inhibitors rely on their abundance of functional groups to attempt to get some electrostatic interactions. It’s essentially throwing everything at the wall and seeing if it sticks.
Unfortunately, this tends to make protease inhibitors not very effective overall as a therapeutic. They’re usually administered as a cocktail therapeutic along with P450 enzyme inhibitors when treating HIV but because of their weak interactions they are easy to escape via viral mutations.
There is a lot of evidence pointing to a lack of protease inhibitor effectiveness against SARS-COV2 already. One of the treatments adopted for standard of care against SARS-COV2 is the administration of a lopinavir/ritonavir regimen. Although a lopinavir/ritonavir regimen was thought to help with treating SARS-COV2 the evidence indicates the regimen is insufficient (papers linked below).
As indicated by Uzunova et. al, 2020:
The efficacy of lopinavir/ritonavir with or without ribavirin is evaluated in SARS-CoV-2 patients under randomized control trials. Currently, it was demonstrated that this combination has no benefits in adult patients with severe Covid-19 [45]. Although protease inhibitors are a common class of medication used in the treatment of HIV-1 infection their efficacy in human coronavirus infections is not convincing.
So it may not even matter whether or not Ivermectin works better than Pfizer’s drug; there’s a good probability that both will not be very effective against inhibiting SARS-COV2’s protease. Instead, it may be more important that these therapeutics show broad spectrum activity.
To expand upon Uzonova et. al, 2020:
Moreover, several anti-HIV PIs are also known to influence other intracellular pathways. It was demonstrated that HIV protease inhibitors (indinavir, saquinavir, and lopinavir), independently from any viral infection, can hinder lymphocyte apoptosis by influencing mitochondrial homeostasis. In view of the weak antiviral activity of protease inhibitors, further studies should be done to ascertain whether the clinical benefit could be attributed to their anti-apoptotic rather than their antiviral activity [46,47].
There are hints that this may be the case with Ivermectin (studies linked below), but more research will be needed to fully flesh out these mechanisms of action as most have been speculated about but have not been tested.
As of now there’s no evidence as of now about Pfizer’s drug being tested for other therapeutic properties so we will need to wait on more information.
Based upon this information it would be hard to argue in favor of any therapeutic to be used as a SARS-COV2 protease inhibitor, which begs the question as to why there is so much hope being put into Pfizer’s drug.
As I indicated in a previous Substack post:
there seems to be this large rush to push several medications through clinical trials even if there are many indications about a lack of effectiveness. Whether this is to help big pharma or is an indication of regulatory capture it doesn’t garner any faith that the general public is being provided a transparent and deeply informative look at what is happening.
I will provide updates as more information comes out, but for now there is strong evidence that Pfizer’s drug won’t be as effective as it is being claimed to be.
Thank you for reading my newsletter. If you enjoy my articles please consider becoming a free subscriber in order to receive notifications.
And share with others who may find these newsletters interesting.
Also, please consider becoming a paid member. The research and work put into these articles takes many hours and being a paid subscriber allows me to continue to do this full time.
Additional Information
Although I intended to add this in the paper it did not seem to flow well so I will post it here.
Ritonavir is being administered with Pfizer’s drug to create a synergistic effect by slowing down the metabolism of Pfizer’s drug. The P450 enzyme that ritonavir works on is the same enzyme that metabolizes Ivermectin. If an area of concern is Ivermectin’s half life it seems reasonable to think that an Ivermectin/ritonavir regimen should be looked into. Apparently this doesn’t seem to be the case and I would hope that it is something that is researched and tested, especially now that there may be evidence that the level of tolerance for Ivermectin is greater than first thought.
Canga, A. G. et. Al, 2008. The Pharmacokinetics and Interactions of Ivermectin in Humans—A Mini-review. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2751445/
This drug is extensively metabolized by human liver microsomes by cytochrome P450. The predominant isoform responsible for the biotransformation of this compound in the liver of humans is cytochrome P-4503A4, converting the drug to at least 10 metabolites, most of them hydroxylated and demethylated derivatives (23).
Sevrioukova, I. F. et al, 2015. Ritonavir analogues as a probe for deciphering the cytochrome P450 3A4 inhibitory mechanism. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4530633/
In-paper Citations
Ferreira, J.C. et. Al, 2020. Biochemical and biophysical characterization of the main protease, 3-chymotrypsin-like protease (3CLpro) from the novel coronavirus SARS-CoV 2. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7747600/
Pavan, M et. Al, 2021. Supervised Molecular Dynamics (SuMD) Insights into the mechanism of action of SARS-CoV-2 main protease inhibitor PF-07321332. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8300928/
Mody, V. et. Al, 2021. Identification of 3-chymotrypsin like protease (3CLPro) inhibitors as potential anti-SARS-CoV-2 agents. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7817688/
Uzunova, K. et. Al, 2020. Insights into antiviral mechanisms of remdesivir, lopinavir/ritonavir and chloroquine/hydroxychloroquine affecting the new SARS-CoV-2. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7444940/
Additional Citations
Lopinavir’s Ineffectiveness
Zhang, X. W. et. Al, 2020. Old drugs as lead compounds for a new disease? Binding analysis of SARS coronavirus main proteinase with HIV, psychotic and parasite drugs. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7126105/
Cao, B. et. Al, 2020. A Trial of Lopinavir–Ritonavir in Adults Hospitalized with Severe Covid-19. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7121492/
Recovery Collaborative Group, 2020. Lopinavir–ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7535623/
Ivermectin’s Possible Broad Spectrum Therapeutic Effects (These are hypotheses and have not been tested yet)
Lehrer, S. et. Al, 2020. Ivermectin Docks to the SARS-CoV-2 Spike Receptor-binding Domain Attached to ACE2. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7652439/
Rizzo, E. 2020. Ivermectin, antiviral properties and COVID-19: a possible new mechanism of action. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7251046/
Indication of Ivermectin’s Antiviral Effects:
Kinobe, R. T. et. Al, 2021. A systematic review of experimental evidence for antiviral effects of ivermectin and an in silico analysis of ivermectin's possible mode of action against SARS‐CoV‐2. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8013482/




Thanks for breaking this down into meaningful chunks. It seems like the push for a miracle drug (just like the miracle vaccine) is again backed by big pharma. Paritaprevir Maybe might potentially be a better choice as it acts on both the His41 and Cys145 residues. There needs to be more real clinical trials and less vitriol about the potential use of Ivermectin.
Or better yet a comparison trial of properly dosed ivermectin vs PF-07321332. That’s what I want to see. Pfizer will never let that happen.