Fluvoxamine- The Little SSRI That Could: Part III
Nonclinical Examination of Fluvoxamine, Sigma-1 Agonism and ER Stress
A Little More on Sigma-1, ER Stress, and COVID
Note: Brace yourselves for another long post! Hopefully it will be the last in this series. If you are unable to view the entire post please check the main hub. Also, please let me know if you feel this information is too dense or too long. I would love to get some feedback!
Hopefully you all have been following along and haven’t been bogged down by all the jargon!
This will be the last MOA that we will examine, and this one will be quite a doozy. It’s probably one of the most important MOAs out of the bunch because of the precarious nature of this biochemical pathway.
Sigma-1 Receptors are ubiquitous and found in critical organs such as the heart, lungs, and the central nervous system. Because of their role in ER stress, we can see why multi-organ failure may be a contributing factor in COVID deaths when vital receptors may be found all over the body.
As stated, the supposed effect of Sigma-1 activation is a calming effect on cells through the reduction of ER stress signaling pathways. This is extremely apparent in animal models where the genes for Sigma-1 receptors are knocked out. In such animal models knockout of the Sigma-1 gene led to increases in cardiac dysfunction (Abdullah et. al.), and suggests that knockout of the gene is likely to cause a host of other organ impairments.
There are 3 main pathways for ER Stress signaling, and each of them may make an appearance in the studies being presented here. Further information will be provided where necessary, but the 3 main ER stress signals are the following sensors:
Inositol-Requiring Enzyme 1 (IRE1)
Protein Kinase RNA-Activated (PKR)-like ER Kinase (PERK- and no, don’t ask me about this naming scheme. Biologists have a really hard time keeping things more...simplified)
Activation Transcription Factor 6 (ATF6)
In short, these 3 sensors are located on the ER membrane attached to chaperone proteins in an inactivated state. However, accumulation of misfolded proteins leads to the eventual UPR and the release of these 3 sensors. Afterwards, the cascade of events attempts to either rectify the misfolded protein issue or possibly lead to cell death.
For an illustration of these signaling pathways, here’s a schematic diagram taken from Almanza et. al.
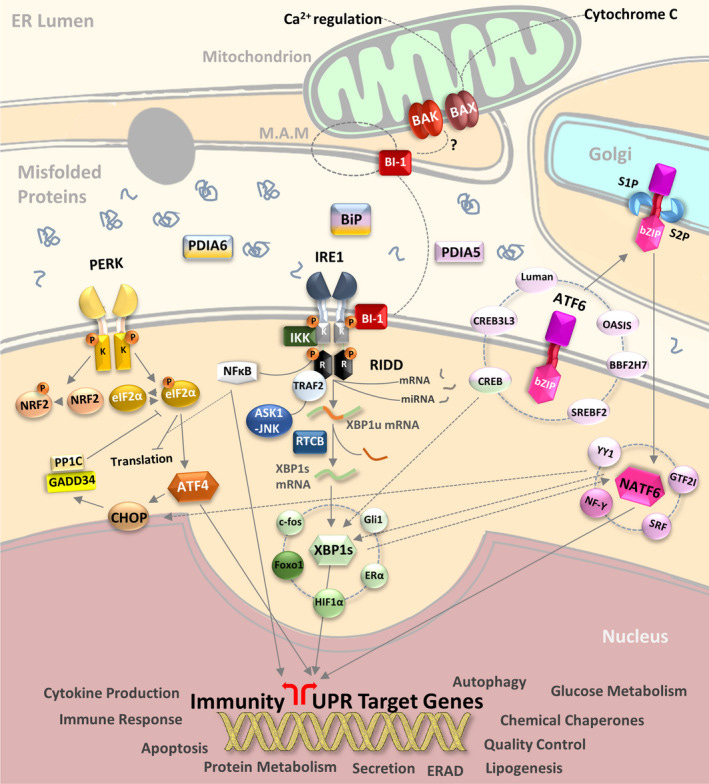
And for additional information check out the following literature reviews (Note: this is not an exhaustive list):
Almanza et. al. : Endoplasmic reticulum stress signalling – from basic mechanisms to clinical applications
Xue, M. & Feng, L.: The Role of Unfolded Protein Response in Coronavirus Infection and Its Implications for Drug Design
What’s important to note is that ER stress has a role in activating the immune system through release of pro-inflammatory biomarkers, signaling through Toll-Like Receptors, and upregulation of nuclear factor kappa B (NF-κB), all of which produce their own inflammatory signaling pathways. Therefore, ER stress itself is likely to play a causative role in cytokine storm production in severe COVID.
In fact, many ER stress biomarkers are elevated in patients infected with COVID. For example, a case-control study from Köseler, et. al. indicated elevated ER stress biomarkers in those with COVID-induced pneumonia. However, elevated biomarkers were also seen in pneumonia patients not infected with SARS-COV2, possibly suggesting a relationship between ER stress and pneumonia as a whole.

Just like with the ASM/Ceramide system, elevated levels of ER stress has been found in many other diseases such as Type II diabetes, neurodegenerative disease, and cardiovascular disease. However, elevated ER stress biomarkers have been seen in those who exercise, although it appears to be attenuated after longterm exercise (Hong et. al.). It’s not quite a surprise that exercise may lead to activation of UPR, as heavy lifting would require the rebuilding and construction of muscle fibers and more blood vessels. Use of Fluvoxamine has also been previously shown to reduce leptin resistance in those with metabolic syndrome, once again validating the association between ER stress and other diseases (Hosoi et. al.). This also explains another association between comorbidities and severity of COVID illness, such that elevated ER stress is likely to play a contributing factor in deleterious disease.
The ER’s role is to essentially serve as a protein production factory for the cell. We can see why, when circumstances require higher production of proteins, that the ER is more prone to making mistakes. Make too many mistakes, and “corrective action” needs to be taken by higher up through UPR signaling. Activation of PERK, IRE1, and ATF6 puts pressure on the cell to correct its mistakes (release pro-inflammatory biomarkers) as well as providing help (chaperone proteins) to clean up the mess of misfolded proteins. If that ends up not working, then the end result is likely “termination” i.e. cell death.
This is the likely explanation as to why so many diseases are associated with ER stress. The need to produce more proteins, whether to increase intramolecular signaling, respond to exogenous substances, or even to construct more protein because of external stress, may lead to the unfolded protein response.
These ideas are reflected in this excerpt from Lin et. al.:
As ER stress and the attendant UPR can lead to cell death, it is not surprising that conditions that lead to an increase in protein misfolding or a decrease in the ability of the cell to handle these proteins in the ER can result in cellular dysfunction and disease. Such diseases may result from the decreased ability of the cell to fold secreted or membrane proteins, the decreased ability to recognize or respond to misfolded proteins, and/or the increased load of misfolded proteins. Inappropriate activation of the UPR may also be harmful, by killing the cell or even by protecting the cell from death (e.g., during neoplastic transformation or viral infection). Indeed, each of these situations, either naturally occurring or experimentally induced, has been shown to cause cellular and/or organismal injury in human beings or in model organisms.
Nonclinical Studies with Fluvoxamine
Fortunately for us, Fluvoxamine appears to have been studies extensively for its role in Sigma-1 signaling. There’ll be a few studies to examine here, but they’ll help to reiterate the importance of the Sigma-1 pathway in disease pathology.
Fluvoxamine alleviates ER stress via induction of Sigma-1 receptor (Omi et. al. 2014)
We’ve outlined above and in prior posts how ER Stress likely plays a large role in COVID, and that the Sigma-1 receptor likely creates a calming effect on both overworked cells and the immune response. Therefore, it would make sense to examine studies that show a direct relationship between Fluvoxamine, Sigma-1 receptors and the attenuation of ER stress.
We’ll start with a pivotal, fairly straightforward study. In this study, researcher examined how Fluvoxamine interacted with Sigma-1 activity and whether that would reduce the effects of ER stress. Because it is a long study I will truncate and select various experiments from this paper. I recommend people read the paper for more information.
Here’s a brief summary of the study:
In the present study, we show that treatment of neuroblastoma cells with Flv induces Sig-1R expression by increasing ATF4 translation directly, through its own activation, without involvement of the PERK pathway. The Flv-mediated induction of Sig-1R prevents neuronal cell death resulting from ER stress. Moreover, Flv-induced ER stress resistance reduces the infarct area in mice after focal cerebral ischemia. Thus, Flv, which is used frequently in clinical practice, can alleviate ER stress. This suggests that Flv could be a feasible therapy for cerebral diseases caused by ER stress.
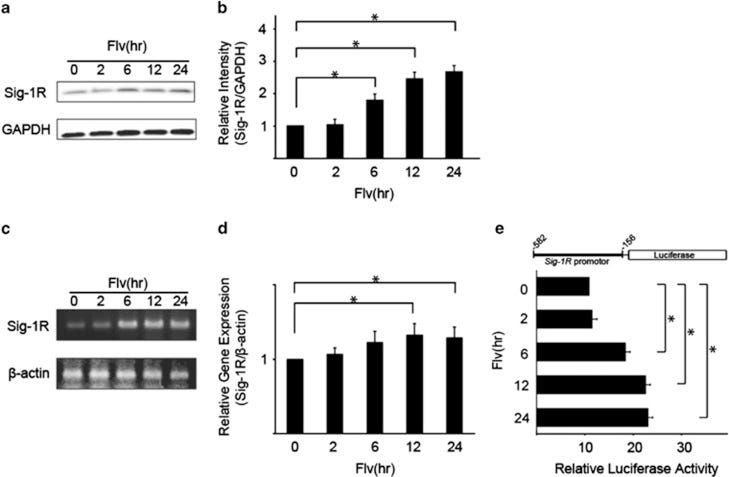
Researchers first wanted to validate that Fluvoxamine acts on Sigma-1 expression. Researchers incubated Neuro2a cells with Fluvoxamine and measured Sigma-1 expression using immunoblotting assays. In an immunoblot assay Fluvoxamine-treated cells are lysed and the Sigma-1 protein is isolated. It is then incubated with antibodies that bind to Sigma-1 and run on a gel and analyzed. The results here suggest that Fluvoxamine increases Sigma-1 expression in a time-dependent manner (Fig. 1A & 1B), in both protein content (Fig. 1C & 1E) as well as transcriptional factors (Fig. 1D).
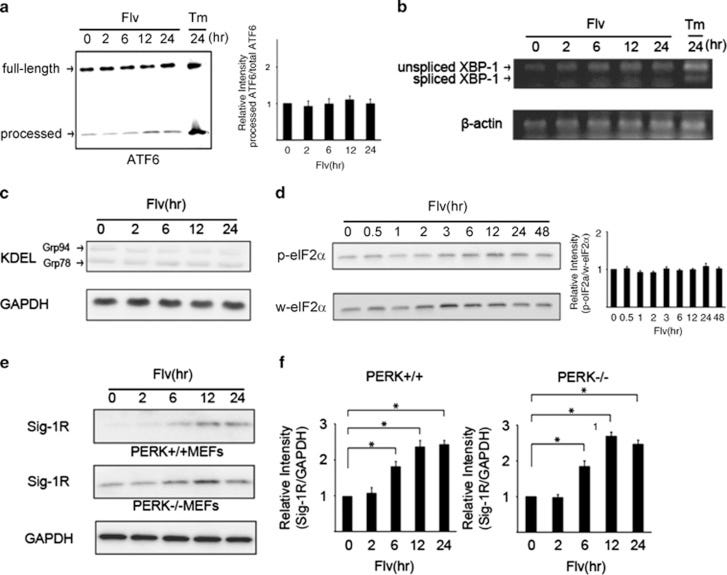
In another study researchers wanted to examine the effect Fluvoxamine had on transcription factors related to the UPR (ATF6, XBP-1, GRP78/BiP, and GRP94). Overall, treatment with Fluvoxamine showed a decline in processed transcription factors, suggesting that Fluvoxamine attenuates the expression of several factors needed to elicit a UPR (Fig. 3A-3D). The researchers also wanted to know if Fluvoxamine itself would activate the UPR through the PERK pathway. However, the results suggest that Fluvoxamine did not activate the PERK pathway (Fig. 3E & 3F). Overall, Fluvoxamine attenuates the UPR without activating the pathway itself.
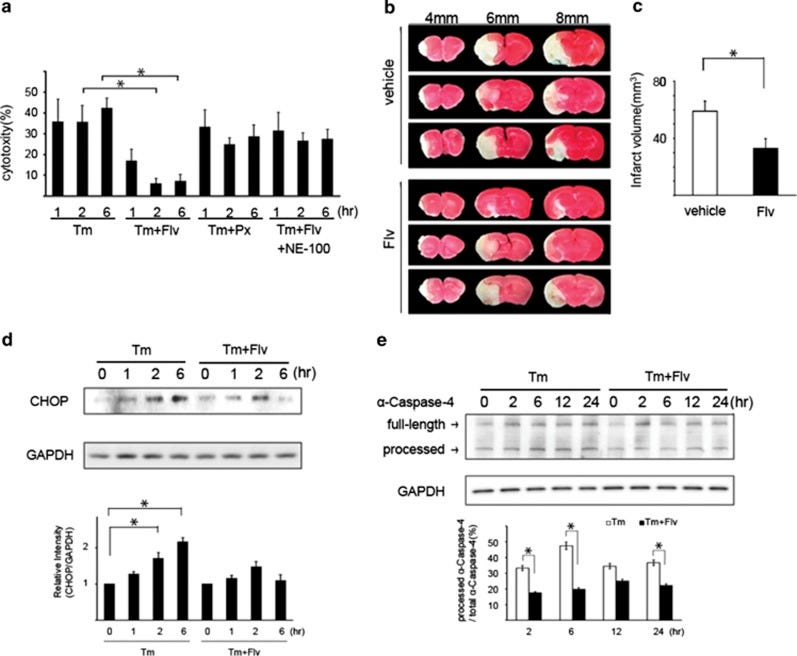
Because excessive ER-Stress leads to cellular apoptosis (essentially cell death), the researchers wanted to examine if Fluvoxamine treatment would reduce ER-Stress induced apoptosis. First, cytotoxicity measures were taken in cells treated with various compounds, such as Tunicamycin (Tm- a commonly used drug for ER Stress induction, sourced from the bacteria Streptomyces), Paroxetine (Px- a commonly used SSRI), or NE-100 (a Sigma-1 antagonist). Overall, Fluvoxamine treatment reduced cell death while treatment with combinations of the other compounds showed no significant reduction, suggesting that Fluvoxamine reduces ER-Stress induced cellular apoptosis through the Sigma-1 receptor (Fig. 5A).
Researchers also examined the expression of two proteins (CHOP and α-caspase-4) known to be indicators of ER-Stress induced apoptosis. Cells were either treated with Tm alone to elicit ER stress or treated with Tm and Fluvoxamine together. When Fluvoxamine was administered, both CHOP and α-caspase-4 activation were hindered (Fig. 5D & 5E). The results here would suggest that Fluvoxamine reduces cell death by reducing activation of ER-stress related proteins.
What’s interesting here is that the researchers mimicked cerebral damage in a mouse model, as cerebral ischemia (brain damage due to lack of blood flow) has been associated with an ER stress response. In order to test the effects of Fluvoxamine on ER stress in the brain researchers used mice and induced middle cerebral artery occlusion (MCAO- essentially cut off bloodflow to the brains of mice) and either provided them a control vehicle or Fluvoxamine. Interestingly, mice who were provided Fluvoxamine showed smaller volumes of cerebral infarct lesions compared to control mice, suggesting that Fluvoxamine may play a direct role in reducing ER stress activity in the brain (Fig 5B & 5C). Considering that ER stress plays a vital role in neuroinflammation, and that cognitive impairments are heavily associated with COVID, we can see that administration of an antidepressant may have ameliorating effects on the brain and CNS as a whole.
Lastly, researchers examined the direct neuroprotective effects of Fluvoxamine through Sigma-1 upregulation. Essentially, does providing Fluvoxamine increase the levels of Sigma-1 receptors, and is it this increase that provides the neuroprotective benefits of Fluvoxamine? The researchers used cell lines that over-express Sigma-1, treated them with Tm and Fluvoxamine, and measured the responses. Once again, the evidence suggests that Fluvoxamine attenuates the ER Stress effects of Tm by increasing Sigma-1 expression (Fig. 4).
This study is highly pivotal to the discussion of Fluvoxamine as it clearly indicates that one of Fluvoxamine’s therapeutic effects are attributed to its engagement with Sigma-1 Receptors. Now, many of these results may appear redundant, but that’s not an entirely bad issue. Redundancy in science can serve as a validation of a hypothesis. Continuous assays that reiterate the fact that Fluvoxamine and Sigma-1 receptors are important for attenuated the ER response, including many of the stress-related biomarkers adds to the validity of such tests:
The results of this study clearly show that Flv, via Sig-1R signaling, induces Sig-1R expression by increasing ATF4 translation, without involving the PERK pathway. In addition, Flv-mediated upregulation of Sig-1R suppresses ER stress-mediated apoptosis to produce a neuroprotective effect. The data strongly suggest that this molecular mechanism offers a new treatment target for diseases thought to be caused by ER stress such as neurodegenerative diseases19, 20 and for diseases associated with reduced Sig-1R levels such as schizophrenia and depression.5, 6
Although the researchers indicate that these results could be used to examine Fluvoxamine in a clinical setting for neurodegeneration, we know that many other diseases are associated with ER stress and Sigma-1 responses, including cardiovascular disease and obesity, suggesting a more broadscale therapeutic effect for Fluvoxamine and other possible SSRI’s through it’s alleviation of ER Stress.
There is a caveat to this study. The bar graph representation for many of these results are derived from the fluorescent immunoblot assays. Whether a computational technique was used to assess the intensity of the gels, the assays should be seen more as a qualitative representation. Unfortunately, many of these assays did not provide quantitative data and so the interpretation of these results may be influenced by bias (I’m sure many of you had difficulty discerning the different intensities between each bar in the gel- I certainly did!).
Nonetheless, I focused on this study solely because it provides the framework to examine Fluvoxamine’s relationship with Sigma-1, and it provides a basis to look into SARS-COV2’s role in eliciting ER Stress. Overall, Sigma-1 Receptors play a vital role in cellular homeostasis, and treatment to maintain such homeostasis may prove beneficial for a host of diseases including neurodegeneration and possibly viral infection.
Modulation of the Sigma-1 Receptor-IRE1 pathway is beneficial in preclinical models of inflammation and sepsis.(Rosen, et. al.)
Going along with the concept of inflammation is sepsis. Sepsis is an overwhelming response to an infection that results in hyper inflammation and multi-organ damage, usually leading to death (taken from Tufan et. al.):
Sepsis is the most common cause of emergency admission to the intensive care unit (ICU) and one of the major reasons for death among hospitalized patients in ICU [1,2]. The first sepsis definition was introduced in the early 1990s, and it has been updated over time by Surviving Sepsis Campaign (SSC) [3]. The current definition alludes to sepsis as a life-threatening organ dysfunction caused by a dysregulated host response to infection. However, the definition of sepsis is a complex issue, and it should be emphasized that sepsis is a clinical syndrome that is determined by the characteristics of both causative pathogens and patients (including such variables as age, gender, genetics, and underlying disease).
The medical terminology for sepsis usually does not take viral infection into consideration as many viral infections don’t tend to cause sepsis. However, the COVID pandemic has reevaluated this concept, as the term sepsis now includes the inflammation commonly seen in severe COVID. The growing concerns over sepsis in relation to COVID would then point doctors to find possible therapeutic options.
This is the scenario seen with Dr. Lenze et. al., who pointed to this study as a reason to try Fluvoxamine in hospitalized COVID patients suffering from sepsis. Therefore, it would make sense to cover the study that led to the first clinical trials of Fluvoxamine.
Because of growing evidence of ER Stress’ role in inflammation, Rosen et. al. decided to examine the role of Sigma-1 receptors in sepsis and what possible therapeutics are available.
I will skip over the first few parts of the study, and refer you to the study for more information. Just know the researchers found that Sigma-1 interacts with the IRE1 pathway (remember that this is one of the signaling pathways for the UPR) by attenuating its signaling (Fig. 1 & 2, not included). Because the IRE1 pathway is involved with cytokine production during the UPR, expression of the Sigma-1 receptor is likely to reduce inflammation.
The first study to examine is one looking at knockout mice. In order to assess the role of SR1 in sepsis, researchers took both control mice (express SR1) and knockout mice (don’t express SR1) and provided them a lipopolysaccharide (LPS) endotoxin and measured their survivability (Fig. 3B). Researchers also examined the effects of fecal-induced periotinitis (FIP- a form of sepsis caused by fecal matter) and saw similar reduction in survivability in knockout mice (Fig. 3E). The results showed a significant reduction in survivability of knockout mice compared to normal mice. When examining pro-inflammatory biomarkers, the researchers also saw greater levels of biomarkers in knockout mice compared to normal mice. Altogether, the results show an important role of SR1 in alleviating sepsis.
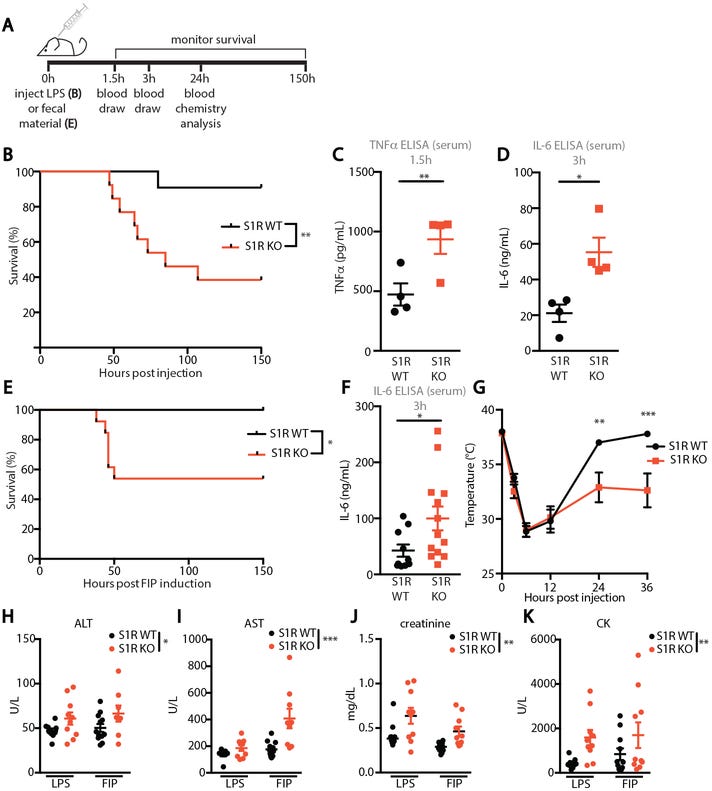
To examine the relationship between SR1 signaling and the IRE1 pathway, researchers provided both knockout and wildtype mice with combinations of LPS, vehicle, and IRE1 inhibitors. Knockout mice provided LPS led to immediate death within that group (Fig. 4C). What’s interesting is that introduction of an IRE1 inhibitor, which would reduce the signaling of that UPR pathway, led to greater survivability (Fig. 4C & 4E). The last result is important, as it indicates that LPS-induced sepsis in knockout mice was due to excessive IRE1 activity. When both knockout and wildtype mice were provided Fluvoxamine, reduction in inflammatory biomarkers was only see in wildtype mice which once again validates the relationship between Fluvoxamine and SR1 in attenuating the UPR (in this case sepsis, Fig. 4F).
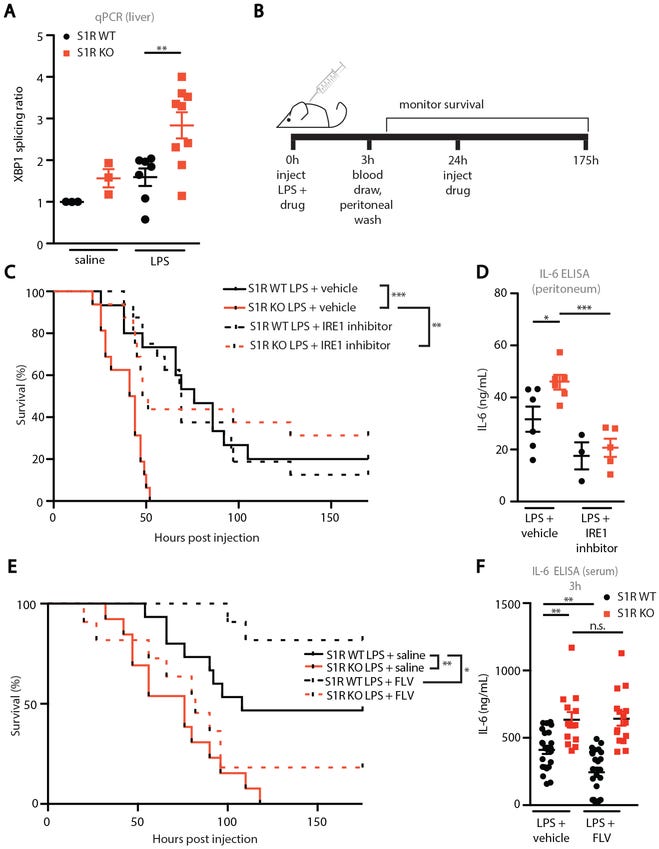
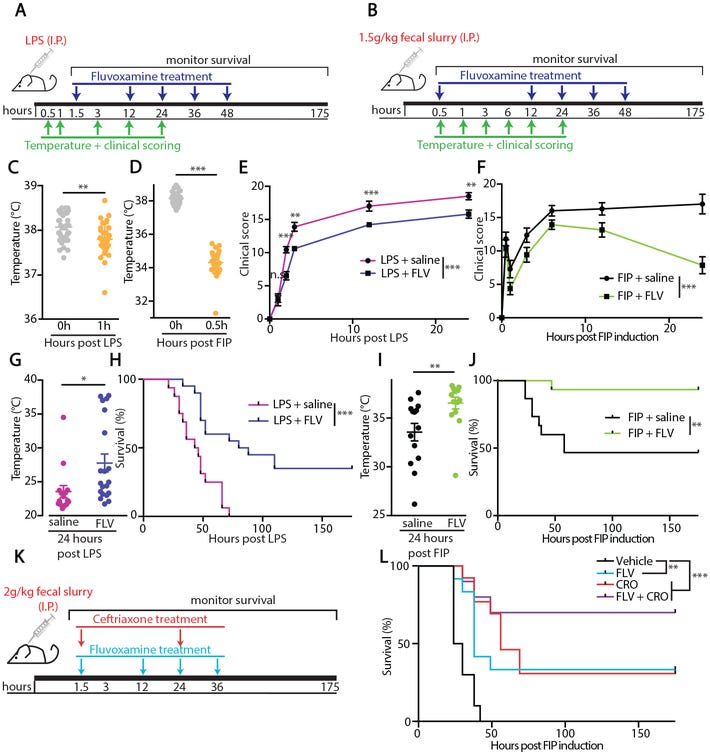
In the last of the animal studies, researchers wanted to examine the effects of a Fluvoxamine therapeutic regimen on mice suffering from LPS-induced sepsis or FIP (Fig. 5A & 5B). Clinical scores and temperature were taken at different time frames to determine severity of sepsis (Fig. 5C-5J, lower clinical scores are associated with improvement). Because FIP may be caused by bacterial infection, researchers provided an antibiotic (Ceftriaxone) either alone or with Fluvoxamine and examined if this dual therapy provided any benefit. The results were not significant, and thus the benefits of Fluvoxamine are likely to be attributed to Fluvoxamine’s anti-inflammatory properties (Fig. 5K & 5L).
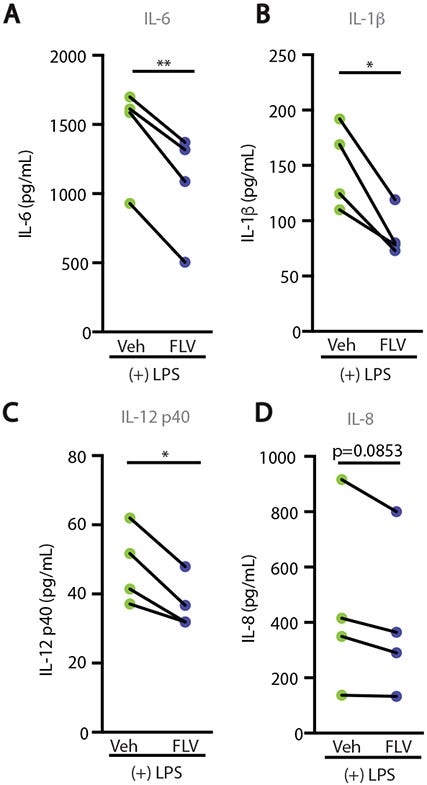
Now, we know full well that animal models are not associated with real-life, clinical scenarios as the same benefits may not be reproducible when translated into humans. Therefore, researchers created one last experiment where they collected blood from healthy donors and treated them with LPS either with or without Fluvoxamine, and then measured the presence of pro-inflammatory biomarkers. The results here showed reduced expression in those treated with Fluvoxamine (Fig. 6A-6D). The researchers considered these results to suggest a conserved SR1 signaling pathway between mice and humans. More importantly, it also suggests that utilization of SSRIs like Fluvoxamine may aid in the treatment of sepsis and other inflammatory injuries. Although this study did not examine regional effects of Fluvoxamine, this study would allude to the idea that systemic inflammation may be attenuated, and thus organ-specific attenuation may not be a concern. We do have evidence from prior studies suggesting that Fluvoxamine is highly neuroprotective, and that levels in the bloodstream may not be as high as within organs.
The importance of this study should not be understated, as it alludes to implications for clinical use of Fluvoxamine against inflammation or sepsis. There’s a reason why this study provoked the examination of using Fluvoxamine in hospitalized COVID patients, leading to the cascade of continuous, ongoing Fluvoxamine clinical trials. This could also possibly explain why clinical trials have mostly focused on Fluvoxamine, since Fluvoxamine was used in this study and was then affirmed in the first clinical trial from Dr. Lenze et. al.
Aside from the typical limitations (low animal sample size, low number of volunteers), an issue with this study is the very high level of Fluvoxamine administered to mice. This study used 20mg/kg of Fluvoxamine. If we assume that the typical American male is 70 kg, that would be nearly 1,400 mg of Fluvoxamine per treatment. If we remember that levels above 100mg may increase the risk of prolonged QT we can see that such a high level may increase the risk of adverse reactions. Whether the researchers were aware of this at the time, it’s unfortunate that heart measurements were not taken. Adding onto this point, the researchers indicated that their ex vivo assay used 20 uM of Fluvoxamine. Without any knowledge of the volume used the mass of Fluvoxamine cannot be determined. Whether it would be an excessive level or not would be up for debate.
Regardless, these two studies are pivotal in examining the pathways between Fluvoxamine and inflammation. In particular, these studies show that Fluvoxamine activates SR1, and it’s through SR1 that the UPR response is attenuated, thus providing evidence of clinical use in COVID. What’s interesting is that Fluvoxamine does not appear to target all 3 of the UPR signaling pathways. The Omi et. al. study indicated that the PERK pathway is unlikely targeted by Fluvoxamine (not covered in this review). Results form the Rosen, et. al. study indicate that SR1 regulates inflammation through targeting of the IRE1 pathway. The evidence is narrow here, and further research may possibly validate or disabuse this idea. Nonetheless, the targeting of 1 UPR signaling pathway should not be overlooked when examined within the broad scope of the UPR.
Where’s the COVID?
Now, at this point you may be wondering why I haven’t covered any studies on COVID yet (or maybe you’re wondering when this post will end?).
Unfortunately, I have not been able to find studies directly implicating Fluvoxamine attenuating SARS-COV2 infection or SARS-COV2-induced ER Stress (essentially preclinical studies of Fluvoxamine treating the ER stress caused by SARS-COV2 infection).
The previous 2 studies suggest that Fluvoxamine increases expression of SR1 and helps to reduce ER stress in that manner. So if we can find evidence of either SARS-COV2 virions or viral proteins inducing ER stress, we may be able to tie together these studies and provide evidence of Fluvoxamine’s direct MOA.
Manipulation of the unfolded protein response: A pharmacological strategy against coronavirus infection (Echavarría-Consuegra et. al.)
It’s funny how many parallels can be drawn between SARS-COV and SARS-COV2 infections. Prior studies have implicated the spike protein of SARS-COV in inducing ER Stress primarily through the PERK pathway (Chan et. al.) Although quite different, we may assume that SARS-COV2’s spike protein may affect the ER Stress signaling pathway as well.
Based on this premise, researchers wanted to determine if SARS-COV2’s spike protein may activate the UPR itself.
First, researchers used prior UPR evidence to examine SARS-COV2’s S, ORF3a, and ORF8 proteins specifically. These proteins were fluorescently tagged and transfected with human embryonic kidney cells (HEK-293T Cells). ER stress biomarkers from each of the 3 UPR pathways were then measured. Amazingly, SARS-COV2’s S activated all 3 UPR signaling pathways (Fig. 4A & 4B, emphasis mine):
ER stress, assessed by the induction of HERP and BiP, was induced by SARS-CoV-2 S but not N (S7A Fig). The PERK-eIF2α-ATF4 branch was activated from 24 h p.t. onwards, as indicated by the phosphorylation of eIF2α and the detection of ATF4 (S7A Fig), although phosphorylation of PERK was not clearly evident. The activation of this pathway was further confirmed by the increase in CHOP transcription compared to mock-transfected cells (S7B Fig). The amino terminus of ATF6 (ATF6-Nt) was detected in S-transfected cells from 24 h p.t. onwards (S7A Fig), indicating activation of the ATF6 branch. Activation of the IRE1α pathway is also evident from an increase in the spliced form of XBP1 in S protein-transfected cells (S7A Fig). Contrary to previous findings for SARS-CoV, this indicates that the expression of the SARS-CoV-2 S protein is sufficient to induce all three major signalling pathways of the UPR.
What’s interesting is that treatment with a UPR inhibitor appeared to reduce the production of UPR biomarkers, and the researchers suggest this may indicate a reversal of UPR activation. The drug used was AEBSF, a serine protease inhibitor that prevents ER Stress-induced cleavage of AT6. Whether similar effects can be seen in SSRIs such as Fluvoxamine or Fluoxetine would be hard to discern due to the drastic differences in MOAs.
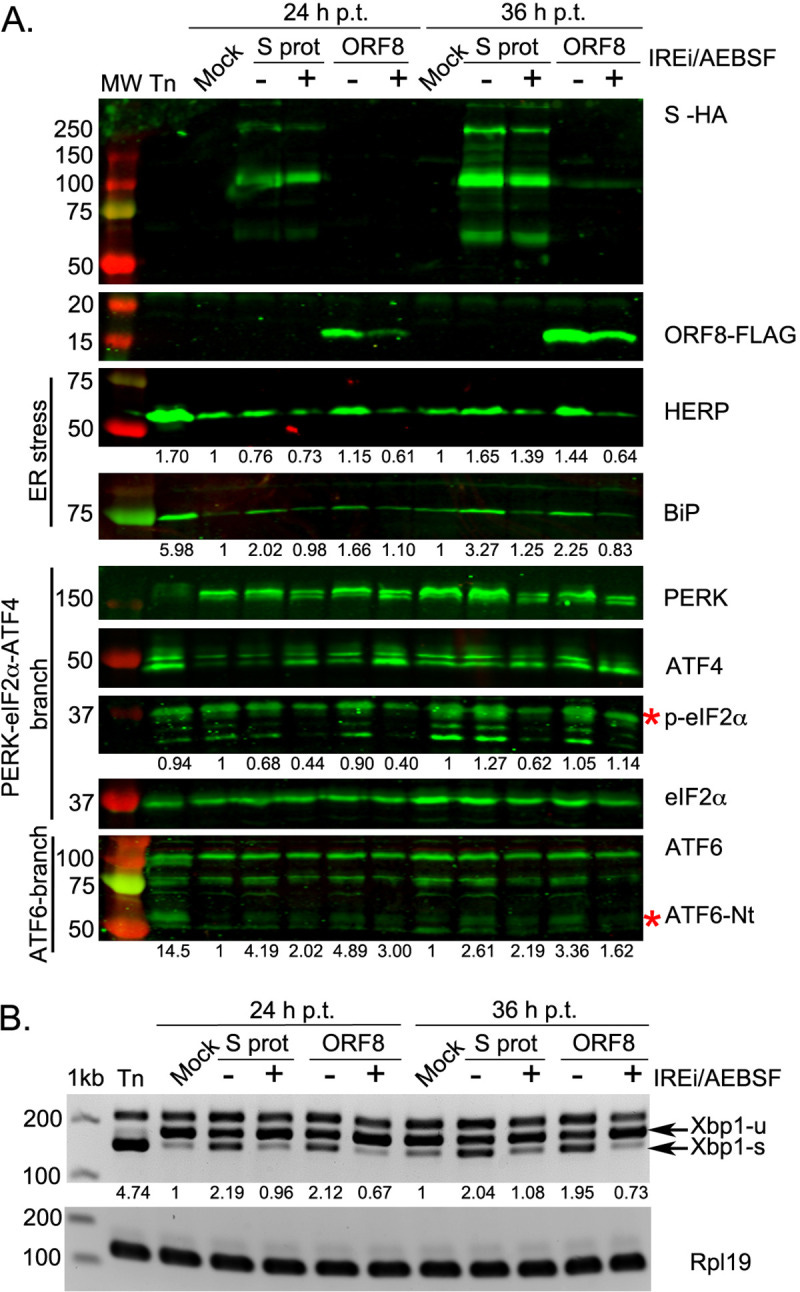
Because evaluation of viral proteins alone is not indicative of an actual viral infection, researchers infected two cell lines (Vero and Calu3 cells, the latter being a better representation of human lung cell lines) with SARS-COV2 and determined the UPR at time points 24 hours and 48 hours post infection. The results here validated those seen in the protein-only analysis, such that UPR was greatly activated by SARS-COV2 infection.
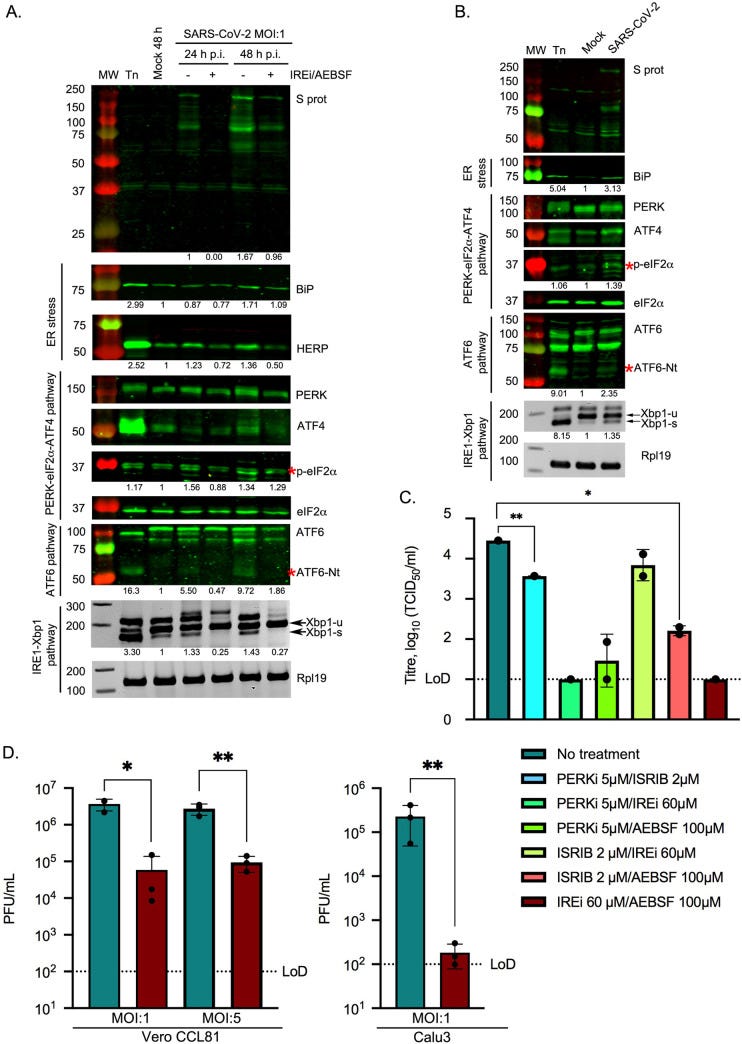
I won’t discuss the rest of the paper as researchers examined UPR inhibitors that target various routes of the UPR pathway and are not applicable here. Just know that UPR inhibition prevented SARS-COV2 replication, in particular by targeting the IRE1 pathway.
Overall, the evidence here shines light on the SARS-COV2’s role in ER stress. Infection by SARS-COV2 induces UPR activation in general, likely through hijacking of host cell machinery to force rampant production of viral proteins. However, the evidence here also indicates that various viral proteins themselves can act directly to alter UPR signaling, most notably by the spike protein which activates all 3 signaling pathways.
This presents with a concerning finding overall. Prior studies have indicated that the spike protein itself can cross the blood brain barrier in mice models and may be a contributing factor in neural inflammation/deterioration in infection (Rhea et. al.), although the relevance to human BBB should be questioned. More notably, the cytotoxic activity of Omicron’s spike protein needs to be extensively evaluated. Prior in vitro studies have indicated that host serine protease does not readily cleave the S1/S2 subunit of Omicron, and that free-roaming S1 subunits may be less likely to occur. This is also reflected in Omicron’s shift towards the endolysosomal route of infection which does not require the cleavage of spike subunits. Whether this shift in infection may reduce the cytotoxic effects of the spike protein alone should be further evaluated, as it may likely explain the reduction in severity along with the shift in tropism.
Adding onto concerns about spike proteins, a preprint from 2020 implicated both the S1 subunit as well as the RBD alone of SARS-COV2 in inducing inflammation, and that the binding of S1 subunits to ACEII receptors may play an active role in the signaling cascade (Hsu et. al.). Questions about the activity of S1/ACEII interactions should be brought up in regards to the extent of binding necessity. Is the “clasping” nature of the S1 subunit necessary for inducing the cascade of events responsible for viral infection and virulence, or can attachment suffice? The results examining RBD binding alone would suggest that conformational changes may not be necessary. It should also be worth noting that lack of the S2 subunit may not provide an anchorage protein to keep all 3 trimeric subunits of S1 together, and thus free-roaming S1 subunits are likely to exist in a monomeric state rather than the typical trimeric state seen on the surface of SARS-COV2. Careful consideration should be taken to include that perspective as well and whether that changes the dynamics of cytotoxicity.
So there may not be direct, nonclinical evidence of Fluvoxamine’s role in SR1 activation during a COVID infection, but we can infer its therapeutic benefits based on the evidence provided here.
Fluvoxamine calms down the ER Stress response by activating SR1.
ER Stress depends upon 3 signaling pathways (ATF6, PERK, and IRE1). SR1 is known to reduce the signaling of these 3 pathways, and thus attenuate the UPR signal.
SARS-COV2, as we have shown above, activates the ER Stress pathways. More specifically, the S protein itself has been indicated to activate all 3 pathways itself. How this occurs is not well known, whether binding to ACEII alone may be enough, or if another pathway is utilized should be taken into account. Nonetheless, the activation would suggest a direct role in altering host cell protein prodcution.
Therapeutics that target the ER stress response greatly reduce viral replication, and upregulation of SR1 has also been show to reduce the UPR. Overall, there are multiple avenues that can be targeted to reduce inflammation as well as viral replication.
So overall we can attest to the high therapeutic value of Fluvoxamine. It’s broad spectrum activity is likely to explain why it is being investigated for COVID, as well as a slew of other diseases including long-hauler COVID from either infection or possibly due to adverse reactions from the vaccines. ER Stress, is its own ubiquitous disease and can be found wherever large-scale protein production is required. Here, we looked specifically into the nature of Fluvoxamine as it relates to ER Stress and how it may alleviate the UPR signaling cascade caused by ER stress.
Hope you’re all still alive! The next post (Part IV) will quickly just cover general in vitro assays of Fluvoxamine and Fluoxetine in cell assays. Then we’ll cover a few pertinent clinical trials and examine the results there. Thanks for holding on! This has taken much longer to both research and post so I hope you are all enjoying in.



Yes, thanks! Any hints or suggestions / previews?
Truly,the use of more than one SSRI at the same time, not sequentially, could ,at least theoretically, be attended by a “stacking” phenomenon, viz. “ 1+1=2.50”. This can be ascertained by experimental observation. What about a SSRI + a SNRI?
My original comment should have been more clear, I.e., what repurposed drugs other than Fluvoxamine seem possibly promising, different types and kinds ?