Evaluating the Evidence Around Molnupiravir (Perspective)
Part I: Examining the Clinical Evidence
I have previously indicated that Molnupiravir may act as a mutagen, and therefore there is a lot of concern about the possible mutagenic effects carrying over to our genome. If that were to happen, we’d have a huge concern about widespread usage of this drug as a prophylactic and early treatment for SARS-COV2.
However, my concerns have always been grounded in an evidence-based, rational approach, and my main concerns are that there doesn’t seem to be widespread studies that assuage the concerns of possible cancer formation with Molnupiravir’s usage.
Nonetheless, there have been many people who have indicated that there has been previous evidence of the dangers of Molnupiravir, indicating that Merck may have known about these concerns about mutagenicity.
Here, I’ll examine the clinical trials, which may point to some possibly concerning ways that the studies were conducted as well as concerns with the data. I’ll also bring into question reports of evidence that a previous drug similar to Molnupiravir may have had mutagenic effects as well.
Evidence in Clinical Trials?
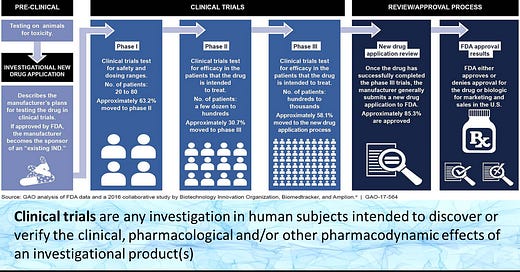
Before we examine if there is any concerning evidence in the clinical trials, we need to make sure we have a basic understanding of how clinical trials are laid out. For that, you can look up any clinical trial chart to get a basic feel but I’ll provide one below to examine.
Pre-clinical:
Pre-clinical trials are based around in vitro and in vivo animal assays that help to examine whether or not a drug may be a good candidate. In the case of Molnupiravir, in vitro assays are intended to examine whether Molnupiravir can inhibit viral infection in infected cells and animal models. They also help to elucidate the possible mechanism of action, as enzymatic assays help to examine whether a drug candidate may target the enzyme in question. In vivo animal models help to indicate whether there may be some concerns with toxicity while also seeing if the drug candidate is effective in an animal model. This also helps to examine whether or not unknown, toxic metabolites may arise.
Phase I:
Phase I trials are not actually intended to examine the effectiveness of a drug candidate against the pathogen/disease in question. Instead, this phase is intended to examine whether there may be any acute concerns with toxicity if the drug candidate was to be administered in humans. It helps to translate further trials into humans by indicating if there are any toxicities that may have been masked in animal models but appear in humans due to anatomical/physiological differences. It also helps to provide a gauge for dose-dependent toxicity if they were to arise.
Phase II:
Here is where the first examinations of possible therapeutic effects occurs. In this phase, people with the disease in question are administered the drug candidate and examined for improved prognosis. In the case of SARS-COV2, researchers may examine if there was a reduction in viral load and hospitalizations in the experimental group versus the placebo/control group.
Phase III:
Phase III and Phase II may see some overlap since both test the therapeutic effects of a drug. However, Phase III tends to have a larger number of participants and also compares the drug candidate to other standards of care. In the case of SARS-COV2, most studies are Phase II/III trials because the therapeutic in question is usually compared to a standard of care therapeutic regimen (i.e. Remdesivir, corticosteroids) as it may not seem ethical to not provide any therapeutic options. A common example of Phase III trials are antidepressants, where the trials may compare a new drug to common antidepressants on the market.
Phase IV:
This phase is not mentioned in the chart, but many therapeutics that are publicly available fall under this phase. The intent of this phase is to see if any long-term effects arise with a therapeutic, usually several years after the drug makes it to market. Usually the notion may be that the benefits of the drug outweigh the risks, although many side effects may not become present until a drug gets longterm, widespread usage. An example of this would be Vioxx, which received FDA approval in 1999 for use in arthritis patients but was removed from the market in 2004 due to its link to heart attacks.
So with this in mind we will examine the pre-clinical and clinical trials for Molnupiravir.
Pre-Clinical Trials
Before a drug candidate is able to reach human trials, it must show strong efficacy in cell cultures and animal testing. What’s important to note is that this would also be a good time to indicate whether or not a drug exhibits cytotoxicity or possible mutagenic activities.
Fortunately, in vitro assays utilizing Molnupiravir indicated strong capabilities of inhibiting viral replication. What’s more important is that animal models didn’t seem to exhibit mutagenic properties after much larger, longer dosing of Molnupiravir compared to what is being used in human trials. The two assays that were used to measure mutagenic effects were the Big Blue and PIG-a assays.
Merck even included information about nonclinical trials in their April press release:
Merck has conducted a comprehensive nonclinical program to characterize the safety profile of molnupiravir. This program included assays such as Big Blue and PIG-a which are designed to provide a robust measure of a drug or chemical’s ability to induce mutations in vivo. Animals were administered molnupiravir for longer and at higher doses (mg/Kg) than those employed in human studies. The totality of the data from these studies indicates that molnupiravir is not mutagenic or genotoxic in in vivo mammalian systems.
This would actually be a good sign that Molnupiravir may not create mutations. But then how do we rectify the Zhou et. al. 2021 piece that indicated mutagenic effects?
The Zhou et. al. 2021 paper I have referenced a lot was conducted in vitro using a higher than typical dose of Molnupiravir and incubated for longer than normal periods. Both the PIG-a assay and the Zhou et. al. Study looked at knockout genes and yet both showed contradictory evidence of mutations.
Although there could be a few factors, one of the likely reasons (and possibly a good one) is that mutagenesis may be dose dependent.
In a cell assay large volumes of Molnupavir may be readily taken up by cells. Because of the high levels of the drug, the level of enzymes (in this case ribonucleotide reductase) may be upregulated in order to convert Molnupiravir from the ribonucleotide form to the deoxyribonucleotide one. Therefore, the high levels of the deoxy form would be likely to be incorporated into cells and cause mutagenesis.
In an animal assay, higher concentrations of the drug are able to disperse and become diluted within the animal model, so even though a higher concentration than what would normally be administered is used, it may not be high enough to affect the conversion of Molnupiravir to a deoxy form. Note that this is a speculation, but that would indicate that something that becomes present in vitro may not not occur in animal models, at least to a noticeable degree.
So would this mean we are out of the woods? Not quite, because unlike other drugs that exhibit cytotoxicity at higher doses, even minor doses of a mutagen can cause damaging mutations to propagate. Mutations in only just a few cells would be extremely concerning, and it’s the reason why this drug in particular should rely on an even greater degree of the precautionary principle.
Far more studies need to be conducted, even if they all result in negative results. This includes Merck releasing the results of their in vivo assays so that they can be examined by the public.
More data means more confidence that mutations are less likely to occur, but as it stands the fast tracked approval process leaves less room for robust evidence that would validate the lack of mutagenesis in humans. In this instance, even more precaution should be taken to ensure that we do not see a possible disaster a few years down the line.
The Clinical Trials
If we look at the clinical trials for Molnupiravir we can notice something concerning. Some of the criteria indicate that no heterosexual sex can occur and that pregnant women must be excluded from the study:
“Males agree to the following during the intervention period and for at least 90 days after the last dose of study intervention: Refrain from donating sperm; and either abstain from heterosexual intercourse as their preferred and usual lifestyle (abstinent on a long term and persistent basis) and agree to remain abstinent; or must agree to use contraception”
“Females are not pregnant or breastfeeding, and at least one of the following conditions applies: Is not a woman of child bearing potential (WOCBP); or is a WOCBP and using a contraceptive method that is highly effective (a low user dependency method OR a user dependent method in combination with barrier method), or be abstinent from heterosexual intercourse as their preferred and usual lifestyle (abstinent on a long-term and persistent basis) for 28 days from the start of study intervention; a WOCBP must have a negative highly sensitive pregnancy test (serum test is required) within 24 hours before the first dose of study intervention”
This indicates a few concerning topics. Sperm undergo one of the highest rates of replication via meiosis, and for a study to indicate that males must not donate sperm and engage in sex indicates that the researchers may be concerned about possible mutagenesis occurring with gametes. There may also be concerns with teratogenic effects, which would explain the reason women who are pregnant have been excluded.
So right off the bat we see some indications that the researchers may be concerned with possible mutagenic effects. To compare, here are clinical trials for remdesivir and ivermectin, indicating no concerns with sperm donation or possible instances of pregnancies within the criteria. It’s strange that the researchers would argue that there aren’t any mutagenic effects and yet will conduct the clinical trials as if there are possibilities. Otherwise, there would be no reason to have listed such a criteria.
Phase I
This section will be quick, as I indicated that the main purpose of this study is to indicate any acute toxicities that may arise.
One of the main Phase I studies was conducted by Painter et. al. 2021:
Here, we report the results of a first-in-human, phase 1, randomized, double-blind, placebo-controlled study to determine the safety, tolerability, and pharmacokinetics of single and multiple ascending oral doses of molnupiravir in healthy subjects. A randomized, open-label, crossover evaluation in the fed (high fat) and fasted states was also conducted to assess the effect of food on the pharmacokinetics of single doses of molnupiravir.
The study was comprised of 64 participants divided into 8 groups, with 6 being administered different doses of molnupiravir and 2 were given placebos.
Measurements of adverse events indicated that more adverse events occurred in the placebo cohort than the experimental cohort, with most of these being mild.
However, in the ascending-dose arm of the study (patients were administered the drug 2x/day) there was one incidence of a severe rash occurring in the treatment group:
One subject discontinued study drug administration on day 4 because of an adverse event of mild, truncal, maculopapular, pruritic rash following multiple BID doses of 800 mg molnupiravir, which was considered by the investigator to be related to the study drug. Following discontinuation, the subject was administered potent topical steroid treatment and antihistamines, and pruritis and rash had both resolved within 18 days.
Strangely enough, the researchers did not indicate what the possible cause of this condition could stem from. It could be an allergic reaction, although it is probably the job of the researchers to examine why such an incident occurred.
Aside from the pharmacokinetics (again, the statistics isn’t quite my strong suite), one of the standing out points comes from the demographics, which seem to be predominately homogenized (similar BMI, male and Caucasian). Although this may not seem like a big issue, it indicates that participant makeup may mask possible differences in pharmacokinetics and metabolism of the drug in different demographics. This was actually a similar issue brought up with the vaccine clinical trials due to the homogeneity of the participants’ demographics, which raised concerns about whether there would be varied effectiveness in different demographics. The same issues may arise here, and the researchers should have attempted to include a wider range of people.
There’s not much else to report on the Phase I trial, although you’ll notice there may be some concerning issues with the next clinical phases.
Phase II/III
As stated above, these phases intend to see whether administration of the drug candidate has any effect on reducing COVID hospitalizations and death.
There were two trials that occurred: one was labeled MOVe-IN where molnupiravir was administered to patients hospitalized with COVID, and the other was labeled MOVe-OUT where molnupiravir was given outside of the hospital setting (these are derived from the April 2021 Press Release).
We’ll start with the MOVe-IN study (emphasis mine):
MOVe-IN (MK-4482-001) was a Phase 2/3, randomized, placebo-controlled, double-blind, multi-site trial evaluating the efficacy, safety, and pharmacokinetics of orally administered molnupiravir in hospitalized participants at least 18 years of age with laboratory confirmed COVID-19 and symptom onset within 10 days prior to randomization. The Phase 2 portion of the trial enrolled 304 participants randomized 1:1:1:1 to who received molnupiravir 200 mg, 400 mg, 800 mg or placebo twice daily for 5 days. The primary efficacy endpoint was to evaluate the efficacy of molnupiravir compared to placebo as assessed by the rate of sustained recovery from randomization through Day 29. Exploratory endpoints supporting dose selection for the Phase 3 portion (Part 2) of the trial included change from baseline in SARS-CoV-2 RNA levels and percentage of participants with undetectable SARS-CoV-2 RNA at various time points, viral RNA mutation rate as assessed by comparison of baseline and post-baseline virus sequencing and pharmacokinetic data (eg, Ctrough, Cmax, tmax, t1/2, AUC0-12). Following an interim analysis of data, it was concluded that the study was unlikely to demonstrate a clinical benefit in hospitalized patients. The decision was made to discontinue the study.
In the April press release Merck indicated that the MOVe-IN trial was ended due to there seeming to be no clinical benefit to those patients hospitalized with COVID.
These results shouldn’t come as a surprise; it has now become more evident that Remdesivir, which was adopted as a standard of care therapeutic for hospitalized patients, was not very effective for helping to reduce severity of illness (the preliminary evidence indicated a reduction from 15 days in a hospital to 12, not a reduction in death). It has become common knowledge that late stage disease progression is dominated by an over reactive immune system that elicits a cytokine storm.
It would be hard to argue that any antiviral would work in such a setting, so the results of this study is not actually shocking. Granted, the study does indicate that endpoints would be based off of viral RNA levels, with the assumption that patients who have reduced viral load would be labeled as having improved while higher viral levels would indicate poorer prognosis. It would be helpful if the researchers elaborated on their protocol so that we can understand what they meant.
It is interesting that they indicate viral RNA mutation rate as a measure. The description seems to indicate that they were measuring for possible viral mutations that may occur over the course of the therapeutic administration, although one would have to question if the viral mutations are suggestive of selective pressure or if there may be concerns about the drug possibly eliciting stronger variants through its mutagenic nature.
Something that’s absolutely shocking about this study is that the control group was given a placebo. In such a setting I find it hard to believe that the researchers did not compare Molnupiravir to standard of care protocols (Remdesivir, Lopinavir/Ritonavir, corticosteroids, etc.). Once again, the researchers did not elaborate on the topic but it seems morally unethical to not provide any type of therapeutic to the control group.
More surprisingly, no data was included MOV-e IN trial, so we don’t even know how long the hospital stay between the two groups are and whether there was any worsening outcomes for the treatment group.
The MOVe-OUT Study
In early October I wrote a newsletter after Merck published an October Press Release indicating the phenomenal results of Molnupiravir. However, I did not examine the information from the press release in detail (that’s my fault) as I was more focused on the biochemistry-related issues. However, there seems to be some concerning features of the study.
Here’s the outline for the study (as indicated in Merck’s press release, emphasis mine):
The MOVe-OUT trial (MK-4482-002) (NCT04575597) was a global Phase 3, randomized, placebo-controlled, double-blind, multi-site study of non-hospitalized adult patients with laboratory-confirmed mild to moderate COVID-19, at least one risk factor associated with poor disease outcomes, and symptom onset within five days prior to randomization. The primary efficacy objective of MOVe-OUT is to evaluate the efficacy of molnupiravir compared to placebo as assessed by the percentage of participants who are hospitalized and/or die from the time of randomization through Day 29.
As I noted with the MOVe-IN trial, the MOVe-OUT trial compares Molnupiravir to a placebo group, and the efficaciousness of Molnupiravir was measures based on hospitalizations or deaths. What’s striking here, again, is that the placebo group seemed to have essentially been offered no other treatments when they were hospitalized.
Merck may have intended to not put all of the trial information into just the press release, but examining this portion on its face seems to point to possibly unethical protocols; it would indicate that patients were essentially allowed to die without being given a treatment option.
Note that the FDA’s website, under its placebo section, indicates that this should not occur:
In clinical trials that include placebos, quite often neither patients nor their doctors know who is receiving the placebo and how is being treated with the experimental drug. Many cancer clinical trials, as well as trials for other serious and life-threatening conditions, do not include placebo control groups. In these cases, all participants receive the experimental drug. Ask the trial coordinator whether there is a chance you may get a placebo rather than the experimental drug. Then, talk with your doctor about what is best for you.
So why was this allowed to happen? Remember that there is no standard of care available for non-hospitalized patients, so it may stem from the lack of a reference therapeutic. Even so, if someone were to progress to severe illness and hospitalization, at that point medical interventions should have occurred since these patients clearly would have counted as having worsened outcomes/prognosis.
On the face this once again seems highly unethical, and for the sake of transparency Merck should clarify what the actual protocols for the study are to disabuse any notion of foul play (and if any of you come across it please let me know so that I can correct it). It’s extremely alarming that deaths were allowed to happen in the first place in the absence of any intervention, and it’s something that Merck needs to elaborate on.
You may have also noticed that many portions of the press release use the phrase “interim analysis”. What that means, as you may have noticed, is that they haven’t examined all of the data yet for this study.
Take a look at this portion (emphasis mine):
The planned interim analysis evaluated data from 775 patients who were initially enrolled in the Phase 3 MOVe-OUT trial on or prior to Aug. 5, 2021. At the time of the decision to stop recruitment based on the compelling interim efficacy results, the trial was approaching full recruitment of the Phase 3 sample size of 1,550 patients, with more than 90% of the intended sample size already enrolled.
It seems the researchers intended to enroll a total of 1,550 patients but only evaluated half (775) of those patients by the time of the Press Release. The most likely reason is that the researchers evaluated the data as patients became enrolled and that was the number they had at the time (which is weirdly half).
What this does mean, though, is that the dataset is incomplete. If they stopped enrolling patients at the 90% mark, that means that nearly 40% of the patients were not yet evaluated and have not been accounted for yet! Even if the addition of that data ends up providing similar results, this data should be taken with heavy skepticism until more results come out.
This alludes to the fault of the media (and of myself as well) for not pointing out this aspect of the press release. The data is not yet complete and yet any mention of the trial indicates the 50% reduction without this in context is extremely misleading; the press release should have been reported with the caveat that more results are going to come in that may alter these results.
Lastly, the study was labeled as a global study, and further in the press release it indicates that there were over 170 trial sites worldwide. This sounds great; it would provide a wide range of demographic data. However, remember that the original intent was to enroll 1550 people, and if we are looking at over 170 testing sites worldwide, that’s an average of 9 participants/site (8/site if we use the 90% enrollment number).
Now, this may be normal (and remember, the 170 are recruitment sites does not mean each one had actual participants), but it would be important to know where these participants came from. Even though this would allow for greater demographics, the highly varied nature may also mean that the results need context to indicate what possible factors could play in the hospitalization and deaths, especially if these were likely to occur in areas that may not have good access to medical facilities or healthcare.
The results of the clinical trials may look good, but they need to be examined with an extreme level of skepticism. The data is incomplete, and there should be concerns of possibly unethical circumstances occurring; clinical trials not allowing medical interventions in worsening patients, resulting in deaths is absolutely an alarming result!
Hopefully soon Merck will release a Press Release with the rest of the data and we can get a better understanding from there, but as it stands some of these results seem extremely concerning.
The second portion will take a look at an expose and see if there was prior history of Molnupiravir’s mutagenicity, which will be released later today.
Thank you for reading my newsletter. If you enjoy my articles please consider becoming a free subscriber in order to receive notifications.
And share with others who may find these newsletters interesting.
Also, please consider becoming a paid member. The research and work put into these articles takes many hours and being a paid subscriber allows me to continue to do this full time.